Early-onset neonatal sepsis (EONS) can cause significant morbidity and mortality, especially if it is not detected early. Given the decrease in its incidence in the past few decades, it is important to find a balance between reducing the use of diagnostic tests and continuing to detect affected patients. We compared 3 detection strategies in patients with risk factors (RFs) for infection: laboratory screening (S1), the Neonatal Sepsis Risk Calculator (S2) and clinical observation (S3).
Patients and methodsRetrospective observational study in neonates born at 34 weeks of gestation or later and with RFs or symptoms compatible with EONS. We analysed outcomes in our unit with the use of laboratory screening (S1) and compared them with the other two strategies (S2 and S3) to contemplate whether to modify our protocol.
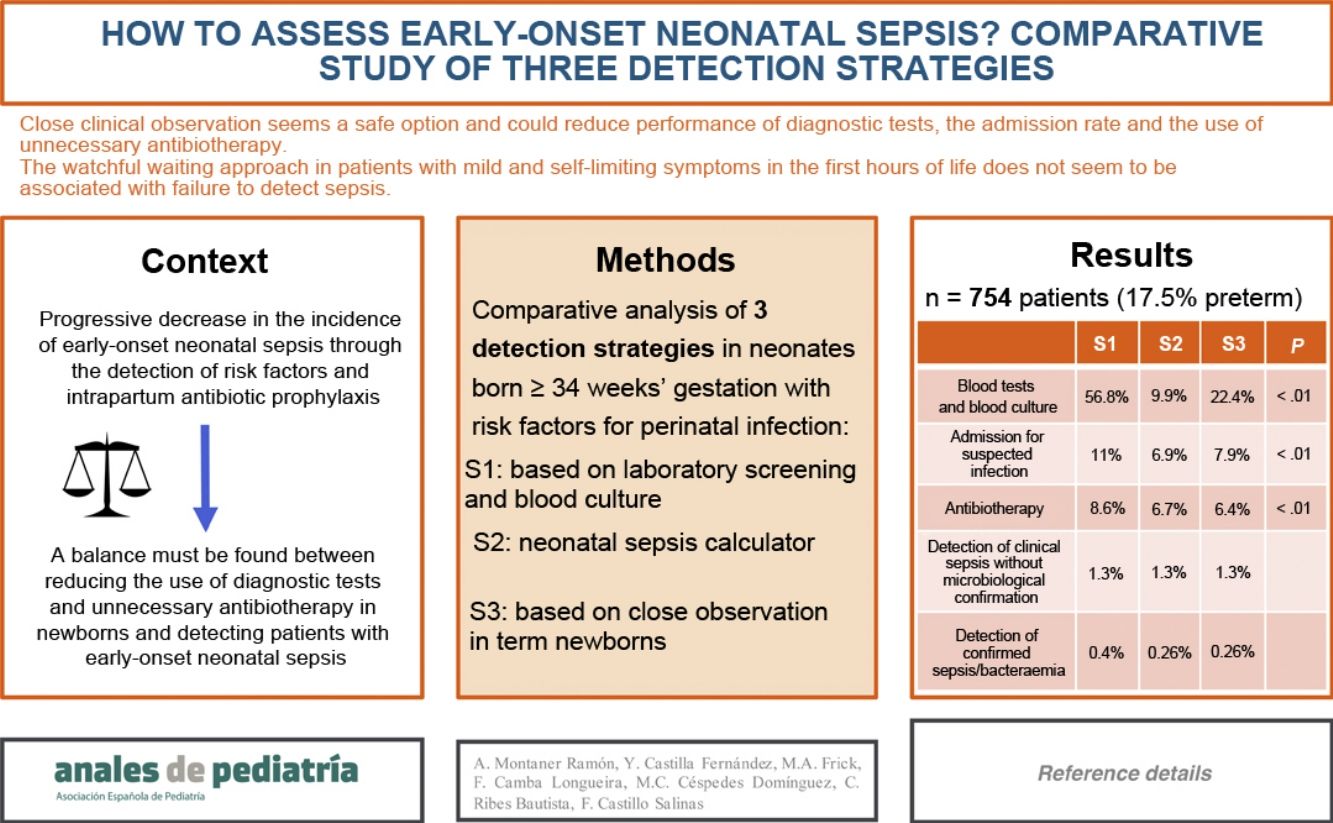
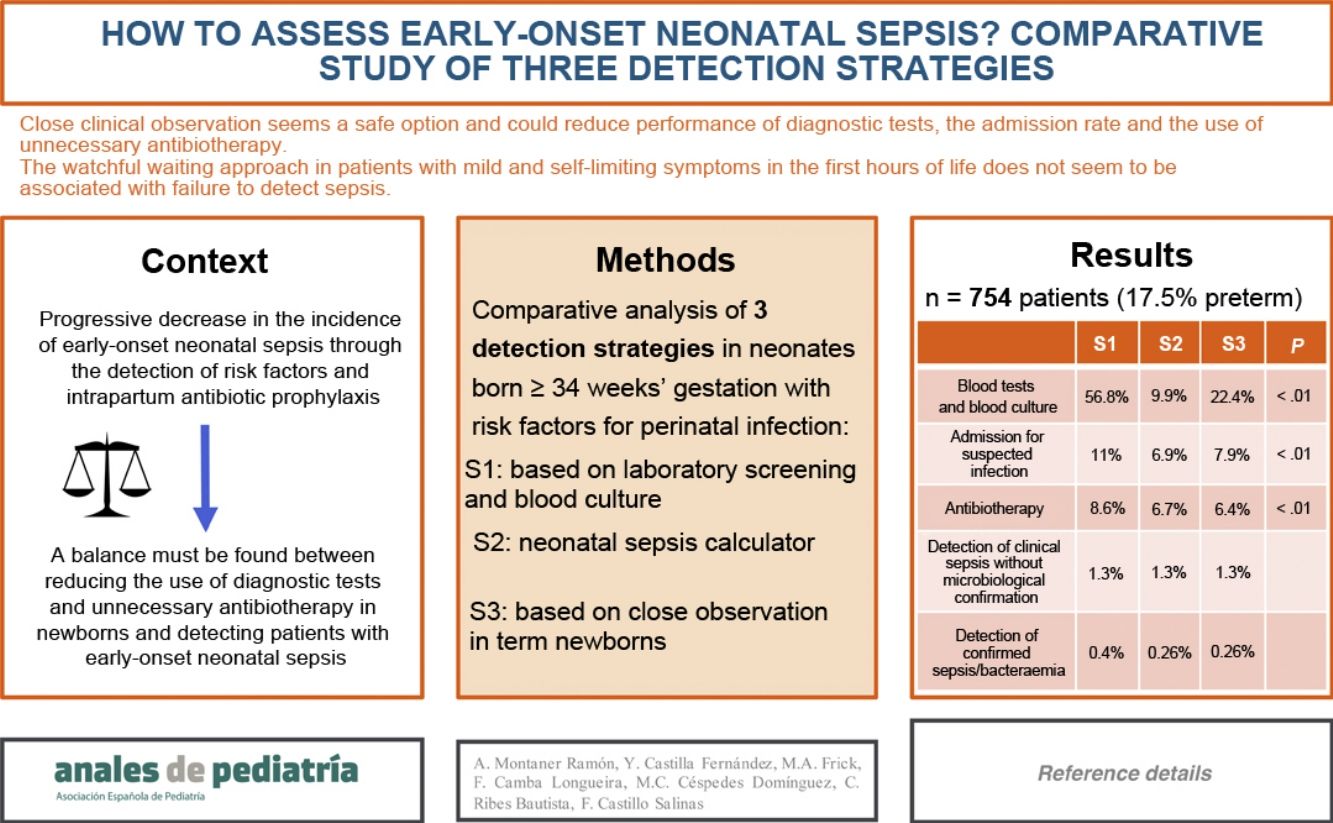
ResultsThe study included 754 patients, and the most frequent RFs were prolonged rupture of membranes (35.5%) and maternal colonization by Streptococcus agalactiae (38.5%).
Strategies S2 and S3 would decrease the performance of laboratory tests (S1, 56.8% of patients; S2, 9.9%; S3, 22.4%; P < 0.01), hospital admissions (S1, 11%; S2, 6.9%; S3, 7.9%; P < 0.01) and the use of antibiotherapy (S1, 8.6%; S2, 6.7%; S3, 6.4%; P < 0.01).
Sepsis was diagnosed in 13 patients, and it would have been detected with S2 and S3 except in 1 patient who had asymptomatic bacteriemia by Enterococcus faecalis.
No patient with mild and self-limited symptoms in whom antibiotherapy was not started received a diagnosis of sepsis later on.
ConclusionClose clinical observation seems to be a safe option and could reduce the use of diagnostic tests, hospital admission and unnecessary antibiotherapy. The watchful waiting approach in patients with mild and self-limiting symptoms in the first hours post birth does not appear to be associated with failure to identify sepsis.
La sepsis neonatal de inicio precoz (SNIP) puede causar morbi-mortalidad importante, sobre todo si se retrasa su identificación. La disminución de su incidencia en las últimas décadas motiva que sea importante encontrar un equilibrio entre reducir las pruebas complementarias y seguir detectando los pacientes afectos. Comparamos 3 estrategias de detección en pacientes con factores de riesgo (FR): E1. Cribado analítico; E2. Calculadora de riesgo de sepsis neonatal; E3. Observación clínica.
Pacientes y métodosEstudio observacional retrospectivo, en recién nacidos con edad gestacional ≥34 semanas y con FR o sintomatología compatible con SNIP. Se analizaron los resultados de nuestra unidad con cribado analítico (E1) y se comparó con las otras 2 estrategias (E2 y E3) para valorar modificar nuestro protocolo.
ResultadosSe incluyeron 754 pacientes cuyos FR más frecuentes fueron la rotura prologada de membranas (35,5%) y la colonización materna por S.agalactiae (38,5%).
Las E2 y E3 disminuirían la realización de analíticas (E1 56,8% de los pacientes; E2 9,9%; E3 22,4%; P < 0,01), los ingresos hospitalarios (E1 11%; E2 6,9%; E3 7,9%; P < 0,01) y la administración de antibioterapia (E1 8,6%; E2 6,7%; E3 6,4%; P < 0,01).
13 pacientes se diagnosticaron de sepsis, las cuales se hubieran detectado con E2 y E3, salvo un paciente con bacteriemia asintomática por E. faecalis.
Ningún paciente con clínica leve y autolimitada en que no se inició antibioterapia, se diagnosticó posteriormente de sepsis.
ConclusionesLa observación clínica estrecha parece una opción segura y podría disminuir la realización de pruebas complementarias, la tasa de hospitalización y el uso de antibioterapia innecesaria. Mantener una conducta expectante en pacientes con sintomatología leve y autolimitada en las primeras horas de vida parece no relacionarse con la no identificación de sepsis.
Sepsis is one of the most common diagnoses in neonatal intensive care units, and early-onset neonatal sepsis (EONS) is defined as sepsis developing within 7 days of birth, although vertically transmitted infections may also have onset later. Its aetiology is most frequently bacterial, and the most frequently involved bacteria in Spain are Escherichia coli (E. coli), Streptococcus agalactiae (S. agalactiae) (which together account for approximately 60% of total cases) and, in third place, Listeria monocytogenes.1,2
The suspicion of EONS is based mainly on clinical features, but these manifestations may be subtle and nonspecific and are also found in non-infectious diseases. Laboratory parameters can help identify it, but they also offer a low specificity and can be altered in other conditions, such as foetal distress or hypoxia-ischaemia.3,4 Blood culture, despite being the gold standard for diagnosis, can have a decreased sensitivity in newborns due to a variety of factors, such as bacteraemia with low bacterial loads, the use of intrapartum antibiotic prophylaxis or difficulty drawing the necessary volume of blood.2,4–6
Universal maternal screening for colonization by S. agalactiae, the improved identification of risk factors for infection and the standardization of intrapartum antibiotic prophylaxis in risk pregnancies have achieved a drastic reduction in the incidence of EONS in the past few decades.7 This has led various scientific societies to consider a change in detection strategies, and the most recent management guidelines published by the American Academy of Pediatrics and several publications suggest that clinical observation of newborns could suffice for screening of vertically transmitted infection.7,8
On the other hand, other tools have also been proposed in recent years, such as the neonatal sepsis risk calculator (available at https://neonatalsepsiscalculator.kaiserpermanente.org), use of which could reduce the rate of hospitalization, the use of diagnostic tests and the prescription of unnecessary empirical antibiotherapy.9–12 However, given the lack of clinical trials of large scope, that it does not take into account the presence or absence of chorioamnionitis and that data on its safety are still scarce, its utility is still under debate, and some authors recommend against its use due to the risk of missing cases of sepsis or bacteraemia or of delayed initiation of antibiotherapy.9,13,14
Delays in the detection and treatment of EONS can cause significant sequelae and even the death of the patient,2 but, on the other hand, the unnecessary use of antibiotics also has deleterious effects, such as altering the normal flora of the newborn, the development of antimicrobial resistance, the increased risk of other infections and even an increase in overall mortality.
In addition, the hospitalization of newborns increases health care costs, results in the performance of more painful procedures and in some instances requires the separation of the newborn from the parents, which can interfere with bonding and initiation of breastfeeding, with the consequent risk to the infant’s neurodevelopment.15 Thus, it is important to find the balance between correctly identifying newborns with EONS and correctly use of diagnostic tests and rational use of antibiotics.
As it seems reasonable to assume that decreases in the incidence of EONS reflect a change in the management of these infants, protocols are increasingly focusing on reducing the use of diagnostic tests and unnecessary antibiotherapy.7,8 The aim of our study was to analyse the efficiency and yield of 3 different screening strategies in the detection of EONS, with the purpose of identify the strategy that offers the best risk-benefit ratio for the newborn, and to assess the safety of the new protocol in our hospital, which promotes a less-invasive approach.
Material and methodsWe conducted a retrospective observational study including neonates born between January 1 and October 31, 2020 at or after 34 weeks of gestation (WG), admitted to the maternity ward or the department of neonatal care who had perinatal risk factors for the development of EONS (Table 1) and/or were admitted due to symptoms compatible with infection.
Risk factors for the development of early-onset neonatal sepsis with vertical transmission.
| Preterm birth (before 37 weeks’ gestation) |
| Prolonged rupture of membranes (≥18 h) |
| Premature rupture of membranes (before 37 weeks’ gestation) |
| Mother treated with antibiotherapy for confirmed or suspected invasive bacterial disease at any time during childbirth or in the 24 h preceding or following delivery |
| Invasive infection by S. agalactiae in previous child |
| Maternal colonization, bacteriuria or infection by S. agalactiae during pregnancy (diagnosis based on culture or intrapartum PCR) or unknown culture status without or with incomplete intrapartum antibiotic prophylaxis |
| Intrapartum maternal fever (≥38 °C) |
| Chorioamnionitis |
| Clinical symptoms(Gibbs criteria): maternal fever AND at least 2 of the following: maternal leucocytosis (>15 000 cells/mm3), maternal tachycardia (>100 bpm), foetal tachycardia (>160 bpm), uterine tenderness, malodorous amniotic fluid. |
| Subclinical features (in absence of fever or other criteria): glucose <15 mg/dL or leucocyte count >30 cells/mm3 in amniotic fluid, presence of microorganisms in the Gram stain, positive amniotic fluid culture |
| Confirmed or suspected infection in twin in case of multiple pregnancy |
Our hospital manages approximately 2700 deliveries a year and has a level IIIC neonatal care unit with 25 intensive care beds and 32 intermediate care beds.16 In addition, there are 4 beds for rooming-in with the mother in the maternity ward for late preterm newborns delivered after 34 WG with birth weights greater than 1800 g in stable condition and for newborns with diseases requiring admission but not intensive care.
We excluded patients for whom we could not obtain full perinatal records due to transfer from another hospital or birth out of hospital.
We defined microbiologically confirmed sepsis by the presence of clinical manifestations of infection with elevation of acute phase reactants and a positive blood or cerebrospinal fluid culture. We defined clinical/laboratory sepsis by the observation of signs or symptoms of infection that could not be explained by a different aetiology associated with elevation of acute phase reactants but with negative microbiological results. We defined asymptomatic bacteraemia by a positive blood culture in the absence of clinical and laboratory features of infection.
We reviewed the electronic health records of each patient to collect data on obstetric variables, risk factors for infection, childbirth variables, clinical features at birth, blood and microbiological tests and neonatal hospitalization-related variables (setting of admission, reason for admission, antibiotherapy [type and duration], length of stay, diagnosis, death, complications).
We collected all the information in a database generated with the software SPSS version 25.0.
When the patients in the sample were born, the old protocol for EONS of the hospital was applied (strategy 1). Based on the clinical manifestations, perinatal data and the risk factors of infection and assuming a probability of EONS of 1 case in 1000 live births based on the incidence in our hospital the previous year, we analysed the hypothetical approach that would have been implemented with the use of the neonatal sepsis calculator (strategy 2) and with the newly proposed protocol of our hospital (strategy 3). Fig. 1 details the characteristics of the three strategies.
We started by performing a descriptive analysis to obtain frequencies and measures of central tendency and dispersion. In the inferential analysis, we used different statistical tests depending on the nature of the variables: Kolmogorov-Smirnov and Shapiro-Wilk tests, χ2 test, Mann-Whitney U and Kruskal-Wallis tests. We then made a comparative analysis of screening strategies, comparing the current protocol (strategy 1) with both the use of the neonatal sepsis calculator (strategy 2) and the newly proposed protocol (strategy 3) in terms of the following variables: patients considered candidates for laboratory screening, hospital admission, antibiotherapy, detected cases and undetected cases of sepsis. We used the McNemar test for paired data. We considered P values of less than 0.05 statistically significant.
ResultsIn the 10-month period under study, 2316 newborns were admitted to the maternity ward or the neonatal unit of our hospital.
After excluding preterm newborns delivered before 34 SG, asymptomatic patients with no risk factors for infection and newborns delivered outside our hospital, the sample included a total of 754 patients. Of this total, 74% were born vaginally and 53.1% were male.
Table 2 presents the frequency of risk factors for infection. One hundred and nine patients (14.4%) had 2 or more risk factors.
Risk factors for vertically transmitted infection in the sample (n = 754).
| n | % | |
|---|---|---|
| Preterm birth (34+0–36+6 weeks) | 132 | 17.5 |
| Prolonged rupture of membranes (≥18 h) | 268 | 35.5 |
| Unknown maternal S. agalactiae colonization status | 159 | 21.1 |
| Maternal colonization by S. agalactiae | 290 | 38.5 |
| Intrapartum maternal fever (≥38 °C) | 79 | 10.5 |
| Suspected chorioamnionitis | 6 | 0.8 |
| Intrapartum antibiotic administration | 491 | 65.1 |
| ≥2 doses: 291 | 38.6 |
Blood tests and blood culture were performed in 428 newborns (56.8%), in 368 as indicated by the protocol of the unit and in 60 due to the presence of symptoms (out of which it would have also been indicated by the protocol in 31).
One hundred and twenty patients (15.9%) required admission (28 rooming-in with the mother, 27 in intermediate care and 65 in intensive care). The reasons for admission were: manifestations compatible with vertically transmitted infection (60 patients), prematurity (n = 24), elevation of C-reactive protein in asymptomatic patient (n = 16), bacteraemia in asymptomatic patient (n = 1) and other non-infectious diseases (n = 19).
Sixty-five newborns (8.6%) received antibiotherapy, of who 12 (1.6%) completed a minimum of 5–7 days on account of clinical/laboratory sepsis (10; 1.34%), microbiologically confirmed sepsis (1; 0.13%) or asymptomatic bacteraemia (1; 0.13%). Three of the cases of sepsis occurred in preterm newborns (2 microbiologically confirmed, 1 clinical).
One patient died at 12 h post birth in the context of septic shock caused by E. coli. The bacterium isolated in the 2 patients with a positive blood culture that survived was Enterococcus faecalis (E. faecalis).
Seventy newborns exhibited symptoms compatible with infection: 10 were not admitted because the symptoms were mild and self-limited to the first hours of life, and 60 required admission, of who 48 received antibiotherapy. In the remaining 12 newborns, a watchful waiting approach was implemented on account of their symptoms being self-limited and compatible with transient tachypnoea or respiratory distress syndrome and the absence of leucocytosis or significant elevation of C-reactive protein (CRP) in the tests conducted at admission.
All of the patients admitted due to clinical manifestations (most of them, respiratory distress), underwent testing of CRP levels. We found statistically significant differences in the maximum CRP levels between the group with a presentation classified as non-infectious (median CRP, 1 mg/dL; range, 0.02–4.63) and the group classified as having sepsis (median 3.87 mg/dL; range, 0.09–10.69) (P = 0.038).
We also found statistically significant differences in the maximum CRP levels between the group of patients who did not receive antibiotherapy and the group that did (no antibiotics, 0.1 mg/dL [0.02–1.38] vs antibiotics, 1.13 mg/dL [0.02–10.69]; P < 0.01).
All patients that received a diagnosis of sepsis or bacteraemia had suggestive symptoms of infection, save for 1 patient with asymptomatic bacteraemia by E. faecalis who underwent screening due to risk factors for infection and whose blood culture prior to initiation of antibiotherapy was negative, a case in which the decision to complete the 7-day course of antibiotherapy was made on account of the characteristics of the pathogen. None of the asymptomatic patients that were admitted to hospital and who started antibiotherapy based on isolated CRP elevation received a diagnosis of sepsis or bacteraemia. Three patients with a diagnosis of sepsis (23% of the cases of sepsis/bacteraemia) had no risk factors for infection.
Table 3 presents the results for the 3 screening strategies.
Comparison of the three strategies for screening of infection in a sample of 754 patients.
| S1 | S2 | S3 | P | |
|---|---|---|---|---|
| Indication for laboratory screening tests and blood culture | 428 (56.8%) | 75 (9.9%) | 169 (22.4%) | <.01 |
| Per protocol | 368 (48.8%) | 109 (14.4%) | ||
| Clinically significant symptoms | 60 (7.9%)* | 60 (7.9%)* | ||
| Admission due to suspected infection | 77 (11%) | 52 (6.9%) | 60 (7.9%) | <.01 |
| Abnormal screening result in asymptomatic patient | 16 (2.1%) | 0 (0%) | 0 (0%) | |
| Clinically significant symptoms | 60 (7.9%) | 52 (6.9%) | 60 (7.9%) | |
| Positive blood culture result in asymptomatic patient | 1 (0.13%) | 0 (0%) | 0 (0%) | |
| Administration of antibiotherapy | 65 (8.6%) | 51 (6.7%) | 48 (6.4%) | <.01 |
| Abnormal screening result in asymptomatic patient | 16 (2.1%) | 0 (0%) | 0 (0%) | |
| Clinically significant symptoms | 48 (6.4%) | 51 (6.7%) | 48 (6.4%) | |
| Positive blood culture | 1 (0.13%)** | 0 (0%) | 0 (0%) | |
| Detection of clinical sepsis without microbiological confirmation | 10 (1.3%) | 10 (1.3%) | 10 (1.3%) | |
| Detection of microbiologically confirmed sepsis/bacteraemia | 3 (0.4%) | 2 (0.26%) | 2 (0.26%) | |
| Undetected sepsis/bacteraemia | 0 (0%) | 1 (0.13%)** | 1 (0.13%)** | |
| Sepsis/bacteraemia cases detected by each laboratory screening performed *** | 0.03 | 0.16 | 0.07 | |
| Screening tests performed to detect a single case of sepsis/bacteraemia*** | 32.92 | 6.25 | 14.08 |
S1, strategy 1 (former protocol of the hospital); S2, strategy 2 (neonatal sepsis calculator); S3, strategy 3 (new protocol).
We found statistically significant differences in the comparison of the former protocol (strategy 1) and the sepsis calculator (strategy 2) in the indication to do laboratory tests (56.8% vs 9.95% of patients with S1 vs S2; P < 0.01), admission due to suspected infection (11% vs 6.9% with S1 vs S2; P < 0.01) and administration of antibiotherapy (8.6% vs 6.7% with S1 vs S2; P < 0.01).
When we compared strategy 1 with the newly proposed protocol (strategy 3), we also found significant differences in the indication to do laboratory tests (56.8% vs 22.4% with S1 vs S3; P < 0 .01), admission due to suspected infection (11% vs 7.9% with S1 vs S3; P < 0.01) and administration of antibiotherapy (8.6% vs 6.4% with S1 vs S3; P < 0.01).
DiscussionOur study demonstrated that the change in the strategy for detection of early-onset neonatal sepsis toward a less invasive approach based on observation could reduce the use of diagnostic tests, the need of hospital admission and unnecessary administration of antibiotics without an increase in the frequency of undetected sepsis.
With the former protocol in our hospital, diagnostic tests were used often, as blood tests and culture were ordered in more than half of patients with a risk factor for infection. In a population similar to the sample under study, the implementation of the newly proposed protocol would result in an important decrease in the laboratory tests performed with the previous protocol, a reduction that would be even larger if the neonatal sepsis calculator was used instead.
With strategy 1, blood culture was indicated in patients with the highest risk (intrapartum maternal fever, suspected chorioamnionitis, prolonged rupture of membranes or preterm birth), as it is the gold standard for diagnosis of EONS, although its use is debatable in the absence of symptoms and elevation of markers of infection, which has led to its elimination in the new protocol in the management of asymptomatic patients.7,17
In our sample, of the patients admitted for initiation of empirical antibiotherapy based solely on CRP elevation in the absence of symptoms, none ended up receiving a diagnosis of sepsis. All had been born to term, and most had 2 or more risk factors for infection. Given the low specificity of abnormal blood test findings and the possibility of acute phase reactant elevation due to non-infectious causes, the more tests are done, the higher the likelihood of admission and of unnecessary antibiotherapy, with the deleterious impact that this may have on the infant and the family, to be added to the health care costs it entails. None of these patients would have been admitted or received antibiotherapy with the new protocol or the neonatal sepsis calculator.
Other earlier biomarkers, such as interleukin-6 (IL-6), have also been studied in relation to EONS. Measurement of this marker had yet to be established in the older protocol of our hospital, so we did not analyse its use in this study, but it has been included in the new protocol. Its high negative predictive value may contribute to a reduction in the performance of additional blood tests and the administration of antibiotherapy if its value in the early stages (in cord blood or within hours of birth) is normal. However, its short half-life diminishes its usefulness as a marker of EONS in patients who are initially asymptomatic, as after the initial hours of infection, its levels could be low. Furthermore, this means that it cannot serve as the sole marker and that subsequent monitoring will have to depend on other markers with longer kinetics, such as C-reactive protein.5,18–20
In both the previous literature and our sample, the factor most frequently associated with the final diagnosis of sepsis was the presence of symptoms, despite their lack of specificity.8,12,21 With the neonatal sepsis calculator, the watchful waiting approach is not contemplated in patients with transient tachypnoea or respiratory distress requiring respiratory support or supplemental oxygen past 2 h post birth, or patients with symptoms in general past this time, and initiation of antibiotherapy is indicated. For this reason, when we compared the 3 strategies, we found that the one in which antibiotherapy would be used the least is the newly proposed protocol of our hospital (strategy 3), which does contemplate close monitoring without initiation of antibiotherapy in these cases. In our sample, none of the symptomatic patients in whom antibiotherapy was not initiated and managed with watchful waiting received a diagnosis of sepsis or required antibiotherapy at a later time.
However, despite observing this decrease in the use of antibiotherapy and that it was statistically significant, considering the clinical outcomes it did not seem so substantial, probably because the use of antibiotics was already restricted with the old protocol. We were unable to assess the reduction in the use of antibiotherapy that would be achieved by measuring IL-6 levels in patients admitted due to the presence of symptoms from the first hours of life because data on this variable were not available. The introduction of IL-6 in the new protocol may contribute to reduce the frequency of unnecessary antibiotherapy further, making the difference more relevant from a clinical perspective.
The older protocol could detect all cases of clinical/laboratory and microbiologically confirmed sepsis diagnosed in the 10-month period under study. Whereas the pathogens most frequently associated with EONS in Spain are E. coli and S. agalactiae, E. faecalis was the most common isolate in our sample, although the proportion of blood cultures that yielded isolates was low.1 Both the calculator and the new protocol would have missed the case of asymptomatic bacteraemia by E. faecalis. This case raised the question of whether the course of antibiotherapy had to be completed, as the patient remained asymptomatic at all times, without elevation of acute phase reactants, and the blood culture of the sample obtained before initiation of antibiotherapy as a control was already negative. Cases of asymptomatic bacteraemia in newborns have been described in the past, but it is difficult to predict whether symptoms of sepsis could develop later if antibiotherapy is not administered, and the literature on the subject is not conclusive, so at that time, the decision was made to complete treatment.17,22,23
In our study, in agreement with the literature, the incidence of sepsis among newborns with risk factors for infection was greater in preterm compared to term infants.11,21 However, although for the time being we believe it would be prudent to continue screening this population with blood tests, all preterm newborns that received a diagnosis of sepsis in our sample exhibited symptoms, and it may be worth considering whether the management of these patients with observation alone would be safe, too.
The main limitation of our study is that we were unable to compare the 3 strategies in the real world, but rather analysed the hypothetical course of action that would have been taken with strategies 2 and 3 in the same sample. Other limitations are its retrospective design, small sample size and low frequency of sepsis, especially of microbiologically confirmed cases. Furthermore, the definition of clinical/laboratory sepsis may have led to counting as sepsis some cases that actually had a non-infectious aetiology.
In conclusion, the change in the management of patients with risk factors for vertically transmitted infection toward a less invasive approached based on clinical observation seems an effective and safe alternative that could reduce the frequency of hospitalization and unnecessary antibiotherapy, although studies in larger sample and, above all, with a prospective design are required to analyse safety data in more depth, especially in high-risk patients, such as late preterm newborns. Watchful waiting in patients with mild and self-limiting symptoms in the first hours of life was not associated with poorer outcomes or undetected sepsis.
Conflicts of interestThe authors have no conflicts of interest to declare.
Previous presentation: this study was presented as an oral communication at the XXVIII Congress of Neonatology and Perinatal Medicine of the Sociedad Española de Neonatología, held online in October 2021.