To determine how many patients may have received a mistaken diagnosis of coeliac disease using the ESPGHAN 2012 and 2020 criteria without performance of biopsy when using chemiluminescence, and to evaluate possible causes of interference affecting anti-tissue transglutaminase antibody results obtained with this technique.
MethodsRetrospective and descriptive study of biopsies of patients with elevated tissue transglutaminase antibodies (tTGA) measured by chemiluminescence in a tertiary care hospital.
ResultsThe sample included 135 patients with a mean age of 7.7 years. The diagnosis of coeliac disease was confirmed in 67 (49.6%) and ruled out in the remaining 68 (50.4%).
Subsequently, among those with a non-diagnostic biopsy, we found that 13 (19.1%) would have met the ESPGHAN 2012 criteria and 17 (25%) the ESPGHAN 2020 criteria. The tTG antibody levels were greater than 10 times the upper limit of normal (ULN) in 27.9%. In patients who tested positive for endomysial antibodies (EMA), the cut-off point for tTGA measured by chemiluminescence that achieved the best combined sensitivity and specificity (Youden index) was 849U/mL (42.5×ULN). On the other hand, if EMA levels were not taken into account, the cut-off point was 301U/mL (15×ULN).
In addition, 16 patients (23.5%) received a diagnosis of chronic gastritis secondary to Helicobacter pylori (HP) infection, of who 50% fulfilled the ESPGHAN 2012 and 2020 criteria for diagnosis without biopsy.
ConclusionsTransglutaminase antibody levels measured by chemiluminescence offer a high sensitivity, but there can be artifacts due to situations such as infection by HP. Therefore, the ESPGHAN guidelines must be revised for application in this particular case.
Determinar cuántos pacientes podrían haber sido diagnosticados de enfermedad celiaca de forma errónea utilizando criterios ESPGHAN 2012 y 2020, si no se hubiera realizado biopsia cuando se utiliza quimioluminiscencia. Así como valorar posibles causas de interferencia en los resultados de anticuerpos antitransglutaminasa mediante esta técnica.
Material y métodosEstudio retrospectivo y descriptivo de biopsias de pacientes con elevación de anticuerpos antitransglutaminasa-IgA (AATG) medidos por quimioluminiscencia, en un hospital de tercer nivel.
ResultadosIncluimos 135 pacientes, con edad media de 7,7 años. Se obtuvo confirmación diagnóstica de enfermedad celíaca en 67(49,6%), descartándola en los 68 restantes (50,4%).
Posteriormente se obtuvo que entre aquellos con biopsia sin alteraciones, 13 (19,1%) habrían cumplido criterios ESPGHAN 2012 y 17 (25%) criterios ESPGHAN 2020. El 27,9% presentaban una determinación de AATG-IgA superior a 10 veces la normalidad. En aquellos pacientes con positividad del anticuerpo antiendomisio (AAE), el punto de corte para AATG medido por quimioluminiscencia que mejor combina sensibilidad y especificidad mediante el índice de Youden es de 849 U/ml (x42,5VN).
ConclusionesLos AATG medidos por quimioluminiscencia tienen una elevada sensibilidad, pero pueden verse artefactados por situaciones como la infección por HP. Por ello, las guías ESPGHAN deberían revisar sus criterios en este caso particular.
Celiac disease (CD) is a systemic immune-mediated disease induced by the consumption of gluten and related prolamins in genetically predisposed individuals.1 Its incidence has increased in recent years2 and it has a variable clinical presentation. The diagnosis of CD in children has been simplified over time, thanks to improvements in serological diagnosis methods and the new diagnostic criteria proposed by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). The most recent ESPGHAN guidelines (ESPGHAN 2020) allow the no-biopsy diagnosis approach in children with serum levels of IgA antibodies against tissue transglutaminase (tTG) 10 times the upper limit of normal (ULN) or greater obtained with a validated test in addition to positive endomysial antibodies (EMA-IgA) in a second serum sample.3 This approach allows rapid diagnosis with a high reliability.1 There is also evidence suggesting that higher tTG titers can predict the degree of villous atrophy in CD.4 On the other hand, the techniques used to measure antibody levels may differ between laboratories and vary in terms of the sensitivity or specificity they offer. The most standardized technique currently available is the enzyme-linked immunosorbent assay (ELISA), but chemiluminescent immunoassay has exhibited substantial diagnostic sensitivity. However, the presence of certain pathogens, such as Giardia,5 may interfere with antibody measurement with the chemiluminescence approach, as can certain autoimmune or inflammatory diseases whose presence may cause a transient elevation of antibody titers unrelated to gluten exposure. A recent study found an association between transient elevation of tTG and the presence of Helicobacter pylori in the gastrointestinal tract.6
The primary objective of our study was to determine the number of patients who may have received an erroneous diagnosis of CD applying the 2012 and 2020 ESPGHAN criteria if a biopsy had not been performed after using chemiluminescence to quantify antibody levels at a tertiary care hospital, and to establish the final diagnosis in these patients. The secondary objective was to analyze the clinical and epidemiological characteristics of the sample to explore possible factors that may interfere with diagnosis.
Material and methodsWe conducted a retrospective and descriptive study of duodenal biopsies from patients with elevated tTG titers measured by chemiluminescence at a tertiary care hospital between 2016 and 2021. The inclusion criteria were a suspected diagnosis of CD, elevation of tTG and having undergone biopsy to confirm or rule out the diagnosis of CD. We excluded patients who had IgA deficiency, with suggestive clinical manifestations but without marker elevation or who only had elevation of gliadin antibodies.
We collected data from the health records on demographic characteristics, the reason for performing the gastrointestinal endoscopy and biopsy and laboratory test results.
Patients with possible CD—those with positive serology but without endoscopic features of intestinal damage—were monitored by means of measurement of serum tTG titers without dietary restrictions. If the titers remained elevated over time, a second endoscopy was performed.
The level of tTG was measured with the INOVA QUANTA Flash h-tTG IgA chemiluminescence immunoassay kit, a method validated for diagnosis of CD3 with an established cutoff of 20U/mL. According to its technical data sheet, this kit offers a sensitivity of 94% and a specificity of 98.1%. In the case of a positive tTG titer, EMA levels were measured in a second serum sample with an immunofluorescence assay that employs monkey esophagus sections as substrate (Viro-Inmun Labor-Diagnostika GMBH).
At least 4 biopsy samples were obtained from the distal duodenum and at least 1 from the duodenal bulb, as established by the applicable criteria. Gastric tissue samples were also obtained in these patients. Duodenal histological changes were interpreted according to the Marsh classification as modified by Oberhuber.7 The diagnosis of H. pylori infection was based on the histological analysis of gastric biopsy specimens.
The statistical analysis was performed using the software SPSS, version 25. We defined statistical significance as p≤0.05.
The study was approved by the Research Ethics Committee of the Autonomous Community of Aragon (CEICA) and adhered to the ethical principles of the most recent revision of the Declaration of Helsinki.
ResultsWe reviewed the health records of 184 patients who underwent gastrointestinal endoscopy for suspected CD due to elevated tTG at a tertiary care hospital between 2016 and 2021. Of this total, 135 met the inclusion criteria for the study, of who 39.3% were male and 60.7% female, with a mean age of 7.7 years (SD, 4.17). When it came to the clinical presentation, 38 patients (28.1%) were asymptomatic, and abdominal pain was the predominant symptom in the sample, documented in 32 patients (23.7%). Eighteen patients (13.3%) had diarrhea and 11 (8.1%) were underweight. The rest of the patients exhibited symptoms such as short stature or weight faltering, asthenia, abdominal distension, dyspeptic bowel movements or amenorrhea as features compatible with CD.
The diagnosis of CD was confirmed in 67 of the patients (49.6%) after the histological examination of the biopsy samples, which revealed Marsh grade 2 or 3 lesions. The sex distribution of these patients was 34.3% male and 65.7% female. Of the patients with confirmed CD, 76.1% presented with compatible clinical symptoms. The mean tTG value was 2241.15U/mL (102×ULN). Three patients in this group had chronic gastritis in addition to duodenal damage.
Thus, in the remaining 68 patients (50.4%), CD was initially ruled out by the findings of the histological analysis. Of these patients, 13 (19.1%) would have met the 2012 ESPGHAN criteria and 17 (25%) the 2020 criteria, and would have received a wrong diagnosis of celiac disease if a biopsy had not been performed. The histological findings in these cases were as follows: 58 (85%) Marsh 0, 10 (15%) intraepithelial lymphocytosis (Marsh 1). In addition, 18 patients in the group in which CD was ruled out were found to have chronic gastritis. Of these patients, 27.9% had tTG titers greater than 10 times the ULN, titers that would be considered significant applying the ESPGHAN criteria. Genetically, in the subset of patients without CD, we found that 53 (78%) were positive for HLA DQ2 or DQ8, 6 (9%) had non-risk HLA alleles and 9 (13%) had not undergone HLA typing.
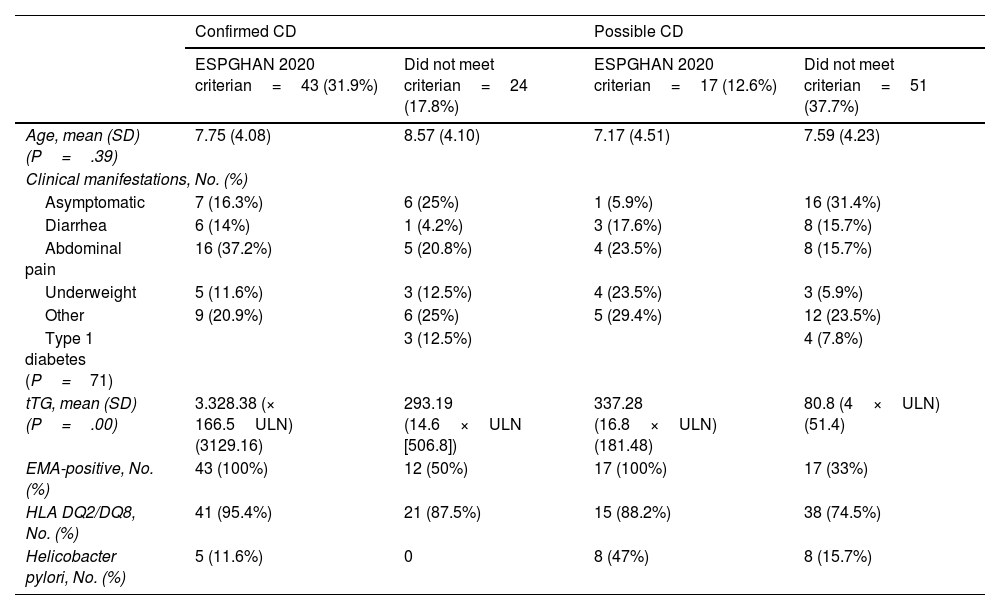
Table 1 summarizes these results, differentiating between cases that did and did not meet the ESPGHAN 2020 criteria.
Summary of the study findings.
| Confirmed CD | Possible CD | |||
|---|---|---|---|---|
| ESPGHAN 2020 criterian=43 (31.9%) | Did not meet criterian=24 (17.8%) | ESPGHAN 2020 criterian=17 (12.6%) | Did not meet criterian=51 (37.7%) | |
| Age, mean (SD) (P=.39) | 7.75 (4.08) | 8.57 (4.10) | 7.17 (4.51) | 7.59 (4.23) |
| Clinical manifestations, No. (%) | ||||
| Asymptomatic | 7 (16.3%) | 6 (25%) | 1 (5.9%) | 16 (31.4%) |
| Diarrhea | 6 (14%) | 1 (4.2%) | 3 (17.6%) | 8 (15.7%) |
| Abdominal pain | 16 (37.2%) | 5 (20.8%) | 4 (23.5%) | 8 (15.7%) |
| Underweight | 5 (11.6%) | 3 (12.5%) | 4 (23.5%) | 3 (5.9%) |
| Other | 9 (20.9%) | 6 (25%) | 5 (29.4%) | 12 (23.5%) |
| Type 1 diabetes (P=71) | 3 (12.5%) | 4 (7.8%) | ||
| tTG, mean (SD) (P=.00) | 3.328.38 (× 166.5ULN) (3129.16) | 293.19 (14.6×ULN [506.8]) | 337.28 (16.8×ULN) (181.48) | 80.8 (4×ULN) (51.4) |
| EMA-positive, No. (%) | 43 (100%) | 12 (50%) | 17 (100%) | 17 (33%) |
| HLA DQ2/DQ8, No. (%) | 41 (95.4%) | 21 (87.5%) | 15 (88.2%) | 38 (74.5%) |
| Helicobacter pylori, No. (%) | 5 (11.6%) | 0 | 8 (47%) | 8 (15.7%) |
EMA, endomysial antibodies; tTG, tissue-transglutaminase antibodies.
In the comparative analysis, we found significant differences (p<.05) in tTG values between the patients with CD and patients with possible CD using the non-parametric Mann-Whitney U test. However, there were no significant differences between the groups in terms of age or symptoms.
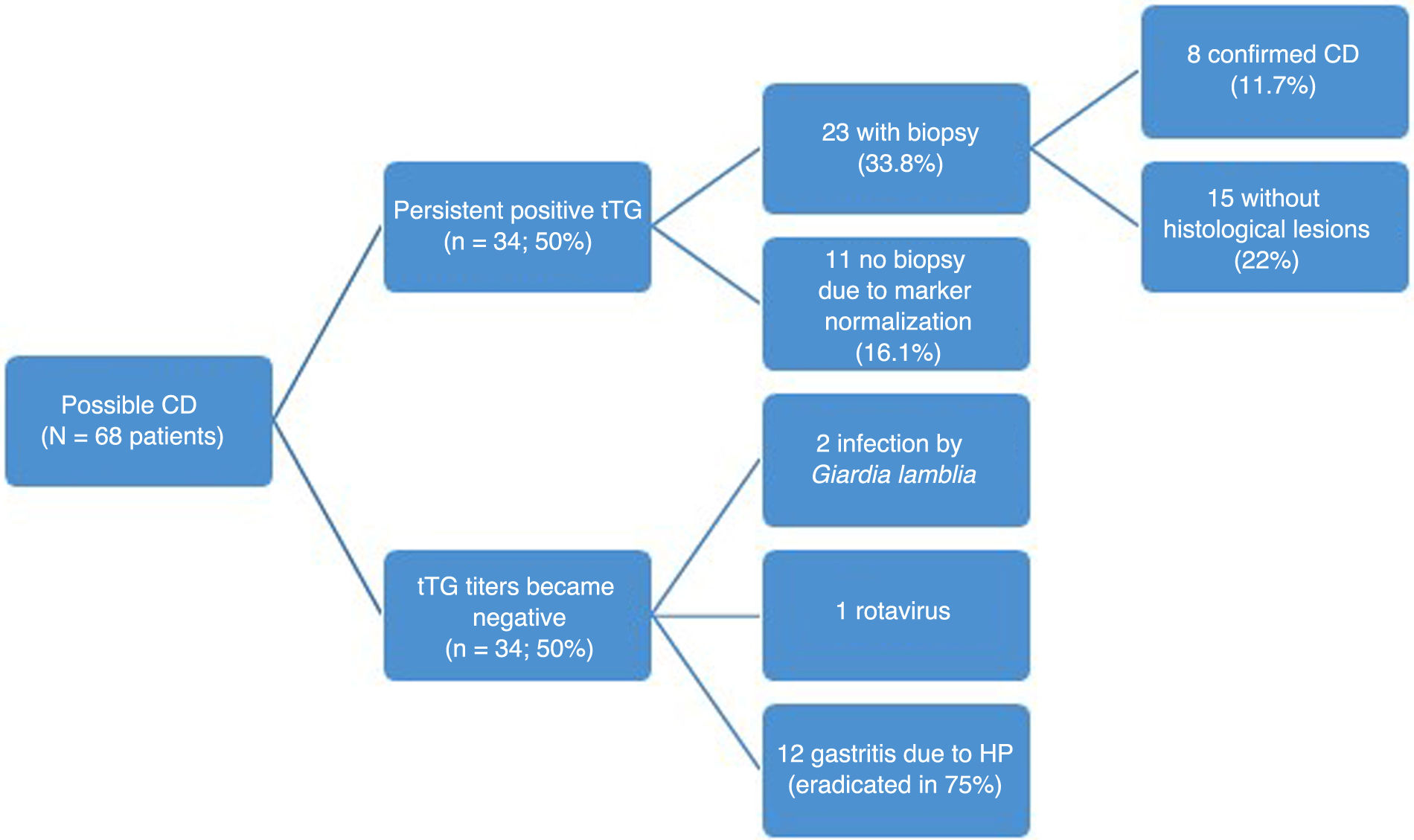
During the follow-up of the 68 patients with potential CD, 34 (50%) continued to test positive for tTG. Of these 34 patients, 23 (67%) underwent a second biopsy due to persistent elevation of tTG, with the histological assessment confirming CD in 8 (11.7% of patients initially not diagnosed with CD). Eleven patients did not undergo a second biopsy, as their tTG titers gradually decreased to values close to the limit of normal and they did not exhibit clear clinical manifestations compatible with CD by the end of the study. The subsequent measurement of EMA titers was not performed routinely in every patient.
In the remaining 50% (n=34) of the patients in whom the initial biopsy findings excluded diagnosis of CD, serum markers eventually normalized. Fig. 1 summarizes the evolution of patients with potential CD.
For the specific subset of patients who met the ESPGHAN 2020 criteria but who did not have CD, we analyzed the outcomes at the end of follow-up. This group included 17 patients, of who 7 (41%) did not undergo a second endoscopy due to the normalization of tTG titers. Nine (53%) continued to test positive for CD markers, out of which six had a second biopsy with no histological abnormalities, two had another biopsy with features compatible with CD later in the follow-up, and one did not have a second biopsy. Serological testing was not repeated in only one of the patients.
Another aspect worth considering is that 16 patients (23%) with possible CD received a diagnosis of chronic gastritis caused by H. pylori. Of these patients, 37.5% were male and 62.5% female. The mean age was 9.25 years (SD, 3.5) and the predominant symptom was abdominal pain, present in seven patients (43.8%). The mean tTG titer in these patients was 218.53U/mL (11 x ULN), with a minimum of 29.2U/mL and a maximum of 846U/mL. Twelve patients (75%) tested positive for EMA, and therefore 50% of the patients with chronic gastritis caused by H. pylori met the 2012 and 2020 ESPGHAN criteria for no-biopsy diagnosis.
When it came to the genetic predisposition of the patients with H. pylori infection,12 (75%) had the HLA-DQ2 haplotype; one (6%) the HLA-DQ8 haplotype and 3 (19%) tested negative for compatible HLA types.
Two patients in the group with possible CD (3%) had infection by Giardia lamblia, and one (1.5%) infection by rotavirus. In subsequent tests following the acute phase of infection, tTG titers normalized, and no further testing was required.
All patients with chronic gastritis secondary to H. pylori infection received first-line eradication therapy with two antibiotic agents and one proton pump inhibitor for 14 days. Once this treatment was complete, tTG titers normalized in 75% of the patients, and the remaining 25% (4 patients) finally received a diagnosis of CD. In regard to the latter subset with CD, we ought to highlight that three of the patients (75%) met both the ESPGHAN 2012 and 2020 criteria at the outset. Based on the results of the rapid urease test, H. pylori eradication was achieved in 75% of the patients.
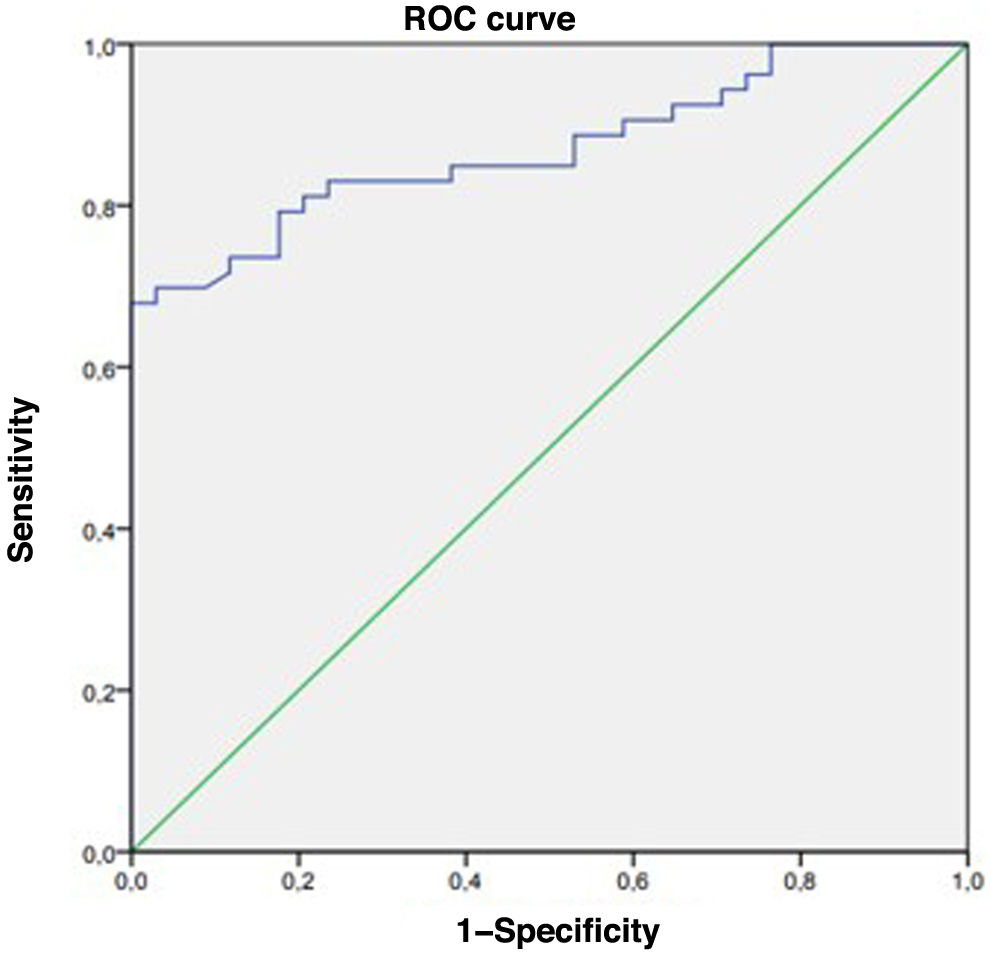
After the analysis of the data was complete, we found that in patients who tested positive for EMA, the tTG cutoff for CD diagnosis with the best combination of sensitivity and specificity according to the Youden index was 849U/mL (42.5×ULN). This was also the lowest cutoff offering a 100% specificity. Thus, in order to make a diagnosis of CD without an intestinal biopsy, in addition to positive EMA, the tTG titers obtained with the INOVA QUANTA Flash h-tTG IgA chemiluminescence assay kit should be at least 42.5 times the ULN (Fig. 2).
DiscussionIn recent years, the approach to the diagnosis of CD has been changing thanks to a growing body of evidence and the improvement in serologic testing methods. Currently, according to the ESPGHAN guidelines, diagnosis without performance of biopsy is possible in some children, minimizing the need of invasive procedures. However, certain conditions have been found to cause transient elevations in tTG titers, one of the required criteria for the no-biopsy approach, which could lead to errors in diagnosis.
In addition, different laboratories use different techniques to measure serum antibody titers. Chemiluminescent immunoassays were introduced a few years ago on account of their high sensitivity and faster turnaround time, which allows their use as a screening method.2,8 They have also proven useful for subsequent monitoring after initiation of a gluten-free diet.2 Due to their high sensitivity, these assays may detect antibody elevation before the development of the characteristic histological changes in the duodenal mucosa. In consequence, some studies that have evaluated the use of this technique have suggested the need to use higher tTG titer cutoffs to apply the ESPGHAN criteria for diagnosis of CE.9 This higher sensitivity stands in contrast to the ELISA approach, which is widely used in a majority of Spanish hospitals.10
In this context, we thought of conducting a study after observing tTG elevation in patients in whom CD had been ruled out to try to identify specific causes for this elevation and determine whether the elevation was persistent or reversible. In this study, we found that higher titers of tTG needed to be applied to achieve a greater sensitivity and specificity in the diagnosis of CD, with an optimal cutoff of 42.5×ULN compared to the 10×ULN proposed by the ESPGHAN. Thus, applying the cutoff identified in our study, 38 patients in the total sample would meet the diagnostic criteria for CD with confirmation of the diagnosis in every case (100%), while, of the 97 remaining patients who would not meet the criteria, 29 would have CD. These results were consistent with those of previous studies, although our cutoff was higher compared to the one proposed by Previtali et al.,9 who found a tTG titer of 560 CU (28×ULN) measured by chemiluminescence in pediatric patients as the cutoff to predict Marsh 2 or greater histological lesions. However, in adult patients, the obtained cutoff was 350 CU (15×ULN).
The high sensitivity of chemiluminescence immunoassays could explain the findings in our sample, as only 49.6% of the 135 patients who underwent biopsy due to tTG elevation received a diagnosis of CD at that time. However, the serology technique employed might not be the sole relevant factor, as several other conditions could contribute to such an increase. Thus, despite the rigor of the studies that provided the foundation for the application of the ESPGHAN criteria, it is important to rule out alternative diagnoses, especially if the tTG elevation is mild.
With regard to H. pylori infection, we found elevated tTG titers in patients with chronic gastritis and isolation of H. pylori from biopsy samples, making this the most frequent etiology in patients in whom CD was ruled out by means of gastrointestinal biopsy. It is worth noting that 50% of these patients met the ESPGHAN criteria for diagnosis of CD. Furthermore, we found that tTG titers normalized in most of these patients after eradication therapy. Other researchers have investigated this aspect, although without differentiating between patients with and without CD, and found that the elevated antibody titers normalized without needing to remove gluten from the diet, thus confirming that H. pylori infection affects serologic testing for CD, independently of the method used (ELISA or the chemiluminescence),11,12 and that the infection could also cause damage to the intestinal mucosa, such as inflammation or crypt hyperplasia. For this reason, it would be advisable to repeat the diagnostic tests after eradication without eliminating gluten.11 However, there have also been studies in which tTG titers did not change after H. pylori eradication.12
When it came to the clinical presentation, the predominant symptom associated with H. pylori infection was abdominal pain, present in seven of these patients (43.8%). Notwithstanding, testing for H. pylori in patients presenting with abdominal pain in the absence of other warning signs or organic features is not recommended, as gastrointestinal pain has a functional etiology in most cases.13 In addition, there is no evidence of an association between CD and H. pylori infection, despite the fact that this pathogen causes intestinal inflammation and histological changes such as intraepithelial lymphocytosis.14 These features can resolve after bacterial eradication. In fact, a meta-analysis on the subject even concluded that H. pylori colonization may offer some protection against CD,15 although the reason for this possible association has yet to be elucidated.
In our study, we identified two patients with infection by Giardia lamblia and one with infection by rotavirus who had elevated tTG titers, so these infections should also be included int the differential diagnosis of CD in patients presenting with compatible symptoms, inflammation without atrophy and tTG elevation.6 Several hypotheses have been proposed to explain why certain infectious agents or inflammatory responses may cause transient increases in antibody titers in genetically predisposed individuals, although the underlying mechanisms are not well understood.16 When such patients have any risk factor for CD (CD in first-degree relative, autoimmune disease, IgA deficiency, Down or Turner syndrome), it is important to be cautious, given the high prevalence of H. pylori or Giardia in certain geographical areas,6 and be watchful for the potential diagnosis of CD at a later time. Thus, if the clinical features are not highly indicative of CD, a possible approach would be to defer biopsy and perform follow-up serological tests to check whether antibody levels normalize.
Genetic predisposition does not offer a high yield, so it is no longer considered a required criterion by the ESPGHAN, since 30% of the general population has CD-associated haplotypes. Eighty-one percent of the patients in our study had the HLA-DQ2 or DQ8 haplotype and only 49.6% of them received a diagnosis of CD, meaning that this costly test with a high negative predictive value (NPV) is reserved for uncertain cases or risk groups.1
The main limitation of our study is that it only included patients who underwent biopsy and excluded those diagnosed applying the ESPGHAN criteria during the same time period, in whom we were unable to determine the tTG levels.
ConclusionThe chemiluminescence method is highly sensitive but less specific than ELISA for measurement of tTG. Consequently, and despite the fact that tTG measurement yields very good and rapid results, it may be necessary to review the ESPGHAN criteria and increase the cut-off value in the particular case of measurement by chemiluminescence to avoid diagnostic errors. In any case, additional comparative studies are still needed.
Furthermore, based on our findings, serum antibody titers measured through chemiluminescence for diagnosis of CD may be affected transiently due to other conditions, such as infection by H. pylori or other intestinal pathogens, which may also cause reversible histological changes. It is important to take this into account in the diagnosis of CD given the long-term repercussions of the dietary restrictions that follow a CD diagnosis, in addition to the presence of any risk factors for CD, which may call for repeated screening.
FundingThis research did not receive any external funding.
Previous presentation: this work was presented at the XVIII Congress of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP).