Primary ovarian insufficiency (POI) carries significant morbidity, causing infertility, sexual disfunction, decreased bone density, cardiovascular risk, emotional distress and early mortality.
ObjectiveTo know the incidence and current management of POI in childhood/adolescent solid tumour survivors.
Material and methodsWe conducted a multicentre observational study. It included female patients aged 12–18 years with a diagnosis of solid tumour and meeting clinical or biochemical criteria for POI. The risk was estimated based on the criteria of the Pediatric Initiative Network of the Oncofertility Consortium.
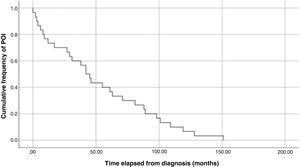
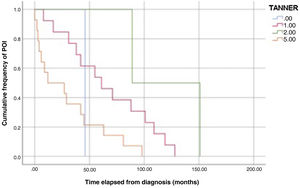
ResultsWe found an incidence of 1.5 (30 cases of POI): The median age at the time of the event was 14 years (standard deviation, 2.09). The solid tumours associated most frequently with POI were Ewing sarcoma and brain and germ cell tumours. Eighty-three percent of patients did not undergo fertility preservation. Sixty-three percent reported not having undergone menarche at the time of ovarian failure. Ninety-seven percent were at high risk of gonadal toxicity, yet 47% were not monitored before the diagnosis. The median time elapsed to the occurrence of the event was 43.5 months after diagnosis and 29.5 months after completing treatment. The Kaplan-Meier curves showed that approximately 30% of POI cases developed within 2 years of diagnosis and that women at Tanner stage 1 developed insufficiency later than women at Tanner stage 5.
ConclusionsThere is room for improvement in the follow-up of women at risk of POI in Spain. The tools currently available facilitate risk assessment at the time of treatment planning and allow the implementation of monitoring, education, early diagnosis, fertility preservation, and replacement therapy as needed. All of this would achieve significant improvement in health outcomes.
La insuficiencia ovárica prematura (POI) conlleva importante morbilidad, causando infertilidad, disfunción sexual, disminución de la densidad ósea, riesgo cardiovascular, alteraciones emocionales y mortalidad precoz.
ObjetivoConocer la incidencia y manejo actual de POI en supervivientes a un tumor sólido en la infancia y / o adolescencia en nuestro medio.
Material y métodosEstudio observacional multicéntrico. Mujeres entre 12 y 18 años con diagnóstico de tumor sólido y criterios clínicos y/o analíticos de POI. El riesgo se estima según los criterios” The Pediatric Initiative Network of the Oncofertility Consortium”.
ResultadosIncidencia de 1.5 (30 casos de POI). Mediana de edad 14 ± 2,09. Los tumores sólidos que más se asociaron a POI fueron: sarcoma de Ewing, tumores cerebrales y germinales. El 83% de los casos no realizó preservación previa al tratamiento. Un 63% no referían menarquia al diagnóstico de POI. 97% cumplían criterios de alto riesgo de toxicidad gonadal, a pesar de ello el 47% no realizó ninguna vigilancia previa al diagnóstico. La mediana de tiempo tras el diagnóstico y la aparición del evento es de 43,5 y 29,5 meses tras finalizar tratamiento. Las curvas de Kaplan- Meier, muestran que a el 30% de los casos, aparecen en los dos años tras el diagnóstico y las mujeres con estadio puberal 1 desarrollan insuficiencia más tardíamente que aquellas con estadio 5.
ConclusionesEl seguimiento de mujeres en riesgo de POI, es susceptible de mejora. Las herramientas actuales facilitan conocer el riesgo al planificar los tratamientos del tumor y realizar vigilancia, educación, diagnóstico precoz, preservación e instauración de tratamiento sustitutivo. Todo ello, supondría importantes mejoras en salud.
Survival in childhood and adolescent cancers has increased in recent years, exceeding 80%.1 However, the sequelae of cancer and the received treatments have a deleterious impact on the quality of life of survivors in adulthood.2 Premature ovarian insufficiency (POI) is one of the possible sequelae. The term POI was proposed by the European Society of Human Reproduction and Embryology (ESHRE) for use in research and clinical practice. The condition is characterised by ovarian failure, deficient ovarian sex hormones and decreased ovarian reserve, which together cause significant morbidity, including infertility, sexual dysfunction, decreased bone density, cardiovascular risk, negative feelings and increased risk of early mortality.3,4
The prevalence of POI in the general population is approximately 1.8%. It is greater in patients with cancer or diseases requiring chemotherapy and/or radiation therapy. In the current literature, some studies have reported that the incidence of POI is 3.5 times greater in girls and female adolescents who are cancer survivors compared to their peers.4 The risk associated with radiation therapy depends on the field and dose of radiation and the age of the patient. When it comes to chemotherapy, alkylating agents are the drugs associated most strongly with POI in every age group.5,6
Close monitoring of ovarian function in girls and women exposed to gonadotoxic treatments is important, as it offers the opportunity of pursuing fertility preservation options, especially in cases in which it was not possible to do so before initiation of treatment. It also allows early initiation of hormone replacement therapy, thereby optimizing health outcomes and quality of life in female patients.7
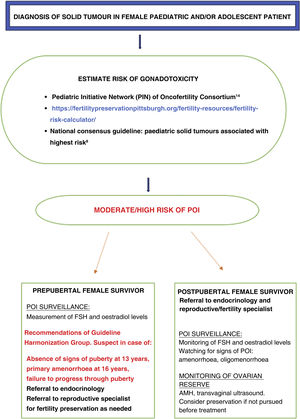
In the case of prepubertal girls, the diagnosis of POI poses a greater challenge. A consensus guideline developed in Spain described the paediatric solid tumours associated with the highest risk of POI.8 In addition, the International Late Effects of Childhood Cancer Guideline Harmonization Group has published evidence-based guidelines regarding the risk of infertility and monitoring of cancer survivors.9 The levels of follicle-stimulating hormone (FSH) and oestradiol are the recommended tests for assessment of prepubertal girls with delayed puberty: absence of physical signs of puberty at age 13 years, primary amenorrhoea at age 16 years or failure to progress through puberty.
The Children’s Oncology Group (COG), the PanCare consortium and other institutions have published guidelines developed by experts for the surveillance and management of adverse events in survivors.10–12 It is essential to raise awareness and educate survivors about the potential adverse events they may develop throughout adulthood. In this regard, girls and female adolescents at high risk of POI and their families should be aware of POI and know how to identify its warning signs and when to consult a specialist. Unfortunately, multiple studies in the literature show that informing patients about the sequelae of cancer and management of these sequelae continue to be inadequate.13
ObjectiveTo describe the incidence and current management of POI in survivors of solid tumours developed in childhood or adolescence in Spain with the aim of improving surveillance, early detection, management and patient education.
Material and methodsA multicentre observational study was conducted by the working group on adolescents with cancer of the Sociedad Española de Hematología y Oncología Pediátricas (SEHOP, Spanish Society of Paediatric Haematology and Oncology).
The study was approved by the ethics committee overseeing research at the Hospital General Universitario Gregorio Marañón under file code GARFOP2021 and conducted in adherence to current regulations, Royal Decree 1090/2015 and Decree 39/94 of the Community of Madrid to uphold the ethics committee resolution.
PatientsInclusion criteriaWe included female patients aged 12–18 years at the time of the study with a diagnosis of solid tumour and a moderate/high risk of developing POI.
Exclusion criteriaWe excluded patients with blood tumours or who had undergone an allogeneic hematopoietic stem cell transplantation.
The study was carried out in 5 referral hospitals in Spain that manage paediatric and/or adolescent patients with cancer. We selected the medical histories of 210 female patients who had solid tumours in childhood or adolescence with a moderate to high risk of POI managed between January 2010 and December 2022 and who had been in follow-up for a minimum of 2 years after completing treatment.
Data collectionWe reviewed the electronic health records of the 210 selected patients, collecting data only for those girls and/or adolescents with a history of cancer that met the criteria for diagnosis of POI. We collected data for relevant variables: age at diagnosis, type of tumour, end of treatment, date of event, history of fertility preservation before treatment or ovarian failure, risk group, menarche, follow-up by endocrinologist, follow-up by gynaecologist, hormone replacement therapy and POI criteria.
We defined the risk groups according to the criteria established by the experts of the Pediatric Initiative Network (PIN) of the Oncofertility Consortium.14
Then, we estimated the risk of POI cases with the following tool: https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/.
We defined POI, in adherence with the recommendations of the ESHRE, as clinical manifestations of menopause with amenorrhoea or oligomenorrhoea lasting at least 4 months and levels of FSH greater than 25 IU/L on 2 occasions at least 4 weeks apart or a level of FSH greater than 40 IU/L at a single timepoint. Measurement of FSH is the gold standard of diagnosis. Levels of anti-Müllerian hormone (AMH) need to be interpreted in relation to FSH and oestrogen levels.15
Statistical analysisData were anonymised upon retrieval in adherence with good clinical practice guidelines before being entered in a database for subsequent analysis, which was performed with the software IBM SPSS version 26 (IBM Corp, Armonk, NY, USA). We summarised categorical data as absolute frequencies and percentages and continuous data as mean and standard deviation. Statistical significance was defined as a P value of less than 0.05. We generated Kaplan-Meier curves to analyse the time elapsed to the development of the adverse event after diagnosis and various factors.
ResultsOf the 210 patients who met the criteria for moderate to high risk, we excluded 170 patients who had not developed POI by the data collection period. We included 30 patients in the study, which corresponded to an incidence of 1.5 in the sample.
The mean age at diagnosis of the tumour was 10 years (SD, 3.88) and the mean age at the time of the event was 14 years (SD, 2.09). As for the type of solid tumour, Ewing sarcoma, central nervous system tumours and germ cell tumours accounted for 70% of POI cases in our study. Eighty-three percent of patients had not undergone preservation of oocytes or ovarian cortex tissue before initiation of treatment and/or development of POI. Sixty-three percent (19) had not gone through menarche at the time ovarian failure was diagnosed. The most frequent stage of pubertal development at the time of the examination was Tanner stage 1 (n = 13) followed by stage 5 (n = 14), which together amounted to 90% of the cases.
At the time of diagnosis of POI, 14 patients had gone through puberty, while the rest were prepubertal and most frequently at stage 1.
A transvaginal ultrasound scan was performed in only 10 patients (33%), and the results were abnormal, with fewer than 10 follicles, in 90%. A transabdominal pelvic ultrasound scan was performed in 23 cases, with normal findings in 11 (37%) and abnormal findings, with visualization of small-sized ovaries and absence of follicles, in 12 (40%).
Hormone level tests evinced FSH levels above 40 IU/L in a single measurement in 78% of the sample and above 25 IU/L in two measurements in the remaining 21%. The level of oestradiol was less than 28 ng/L in 90% of cases. The level of luteinizing hormone (LH) was elevated in 25 measurements (83% of tests). Measurement of AMA levels was not considered necessary for diagnosis. It was carried out in 7 patients (23%) and found decreased values in all.
Sixteen patients (53%) exhibited symptoms of menopause and 23 (77%) had oligomenorrhoea or amenorrhoea of at least 4 months’ duration.
When it came to the risk factors for POI, 20% (n = 8) had received alkylating agents at intermediate doses and 53% (n = 16) at high doses. Thirteen (43%) had received high-dose chemotherapy followed by haematopoietic stem cell transplantation as consolidation therapy. Forty percent of patients received platinum-based antineoplastic drugs. Eight patients (27%) received abdominal irradiation at a dose greater than 10 Gy. Six patients (20%) received cranial irradiation at a dose greater than 40 Gy. Five patients (17%) underwent oophorectomy.
Combining the different risks in each of the patients included in the sample according to the criteria described in the material and methods section and using the risk calculator, 97% of patients were at high risk of POI and the rest were at moderate-to-high risk.
Concerning the monitoring of adverse events recommended in this at-risk population, we found that14 patients (47%) were not followed up by an endocrinologist, 5 (17%) had attended 1–5 follow-up visits at irregular intervals, and 11 (37%) were being monitored for adverse events by endocrinology and/or gynaecology care teams.
A paediatric oncologist made the diagnosis of POI in 33% of the patients, and in many of the other patients made a referral to a gynaecologist and/or endocrinologist. At the time of the study, 26 of the patients (87%) were receiving hormone replacement therapy.
The median time elapsed to the occurrence of the adverse event was 43.5 months from the diagnosis of the tumour and 29.5 months from the completion of treatment.
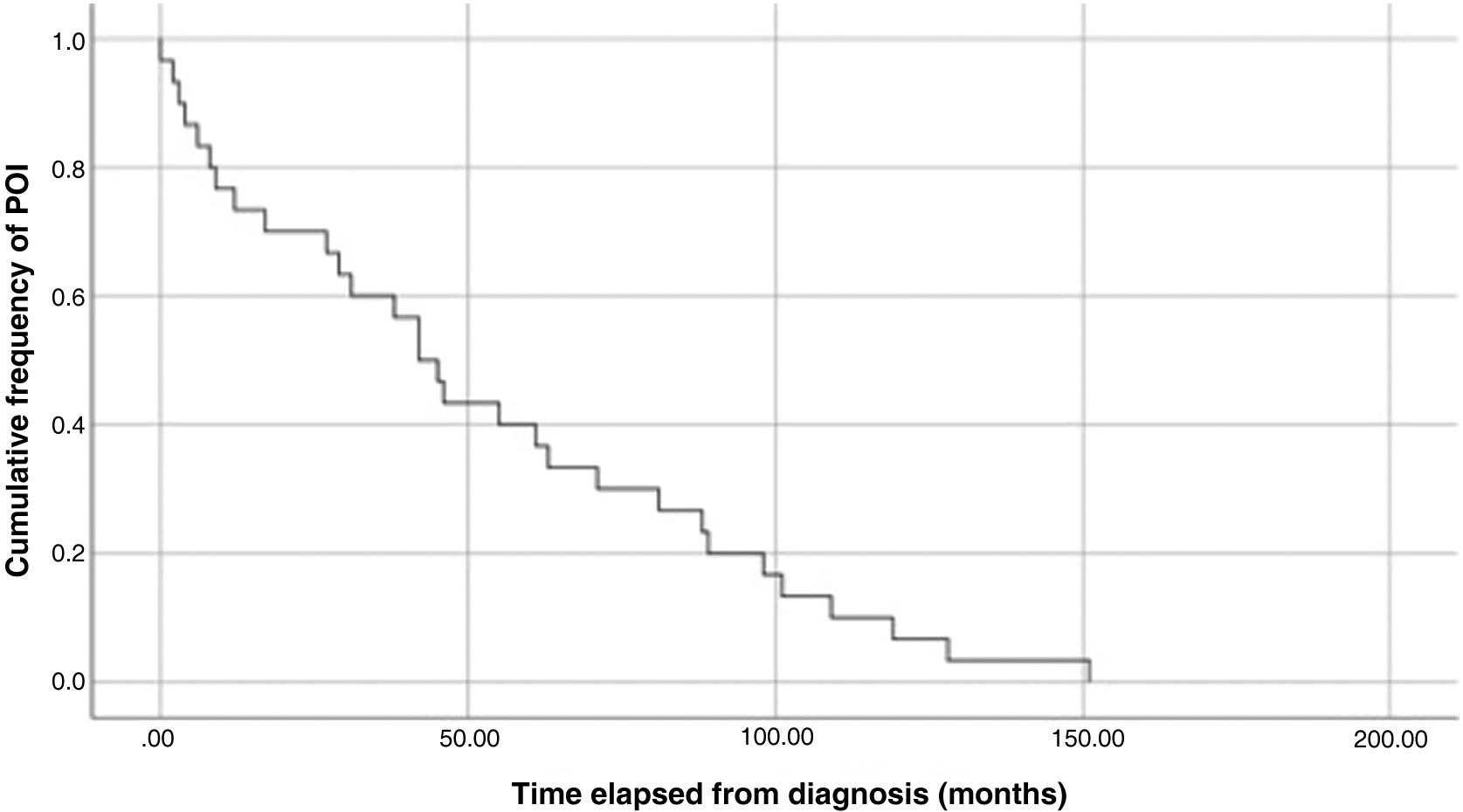
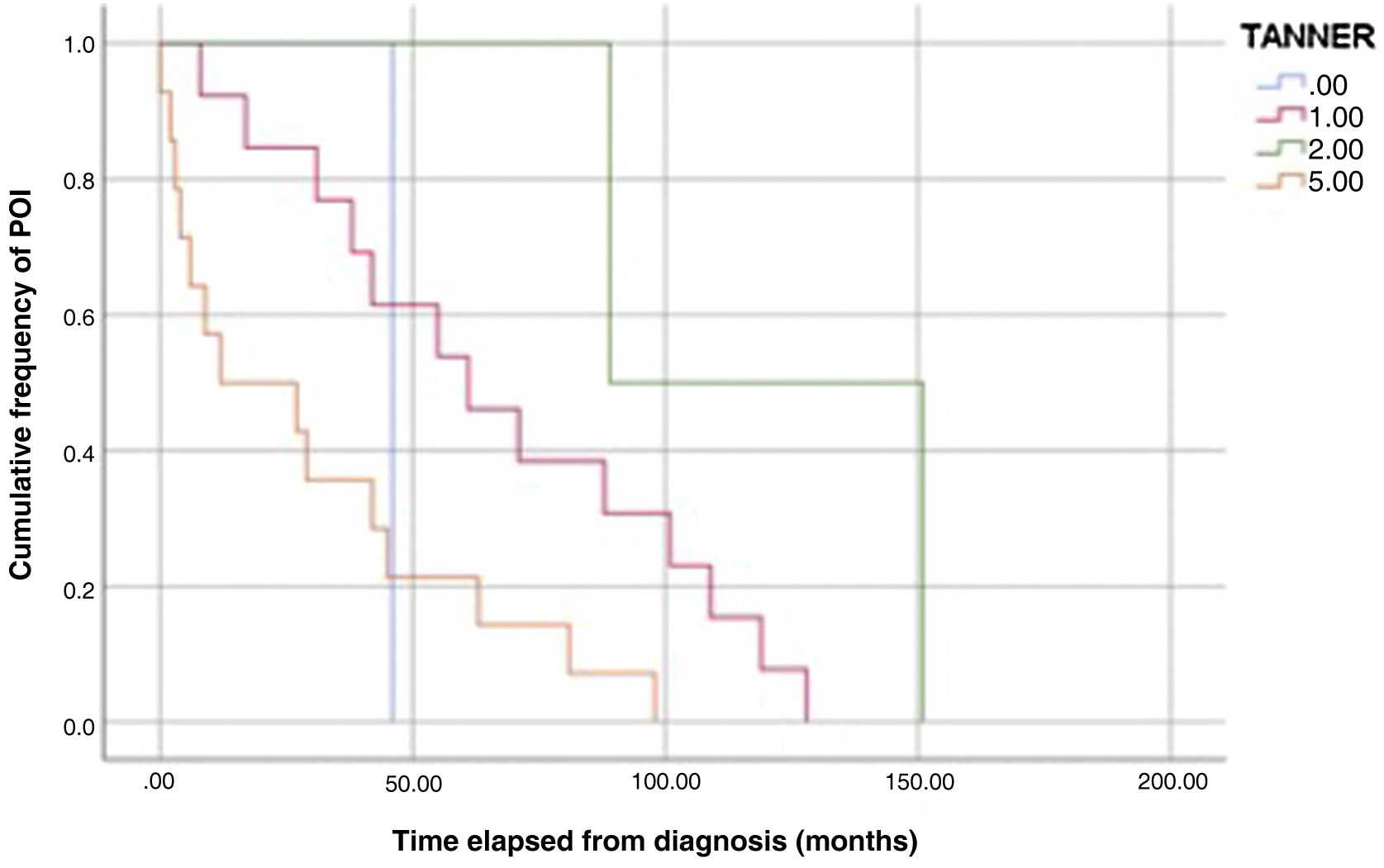
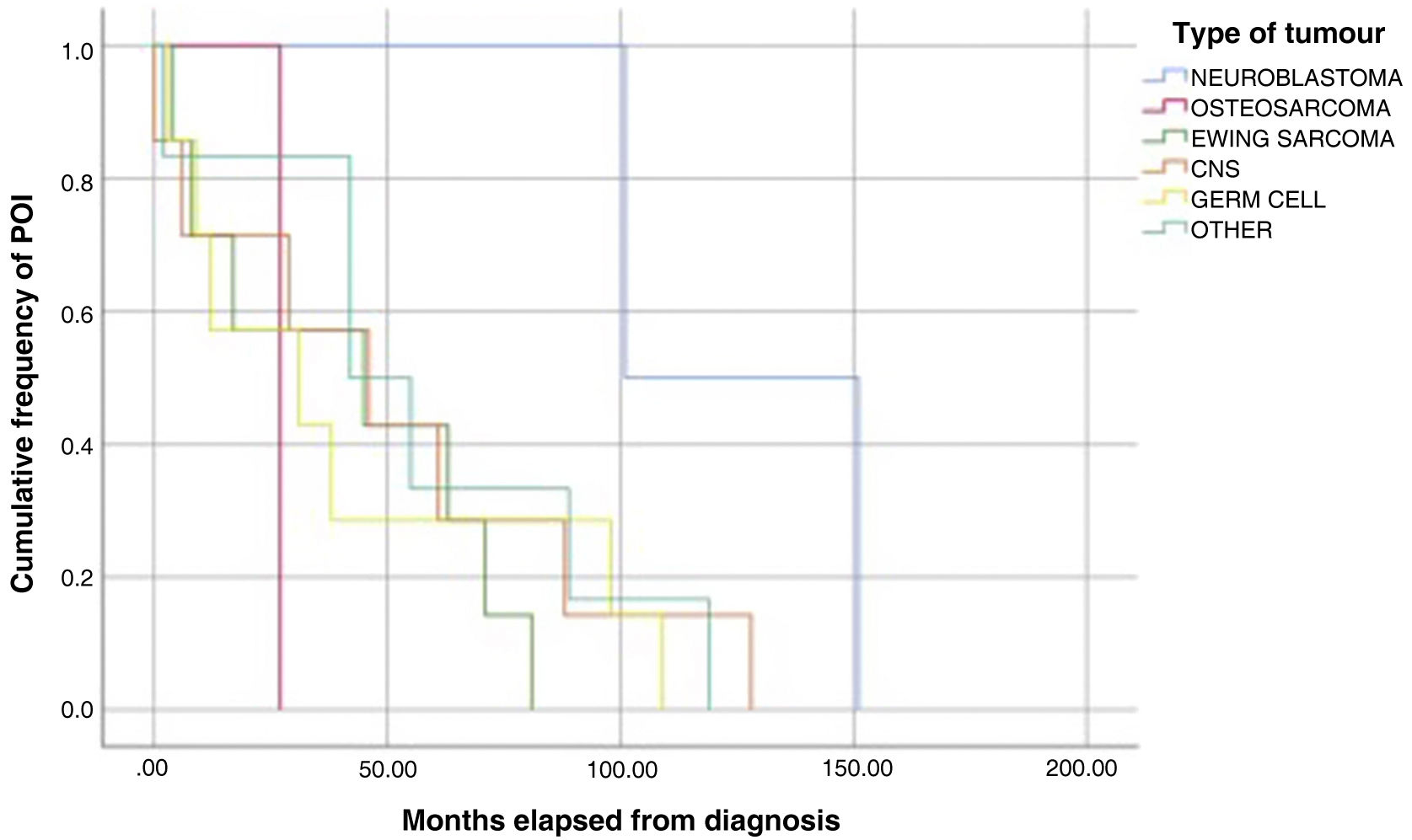
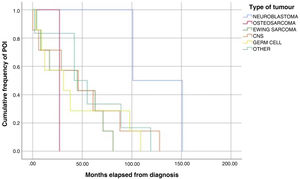
The analysis of Kaplan-Meier curves (Fig. 1) showed that approximately 30% of POI cases developed within 2 years of the diagnosis of solid tumour and some cases had late onset several years after the diagnosis. When the Tanner stage of development was included as a factor in the same analysis, we found significant differences in the development of POI between prepubertal (Tanner 1) and postpubertal patients (Tanner 5) (Fig. 2). On the other hand, we found no differences based on the type of tumour (Fig. 3).
As the number of childhood/adolescence cancer survivor increases,16 the importance of the management of long-term adverse events cannot be neglected. The gonadotoxic effect of treatment has been identified as one of the greatest concerns of childhood cancer survivors and their parents.17 It has also been found to have a deleterious impact on the quality of life of childhood cancer survivors.18
The risk of POI in the general female population is approximately 1%.19 This risk increases in childhood and adolescent cancer survivors, with a prevalence of 5.4% in adolescents with a history of cancer compared to 2.2% in the general adolescent population.20,21 The risk of developing POI before age 40 years in childhood/adolescent cancer survivors is variable. Different studies have found cumulative incidences ranging from 8% to 10.9%.22,23 In our study, we found a cumulative incidence of 1.5%. We ought to underscore that POI may develop in the paediatric age group following cancer treatment, so monitoring should be initiated early, as the risk of POI increases progressively in the following decades until peaking at approximately 34 years of age.23,24
In our study, the median time elapsed to the occurrence of the event was 43.5 months from diagnosis and 29.5 months from completion of treatment. The Kaplan-Meier survival curves show that 30% of cases of POI developed within 2 years of diagnosis of the tumour and that some cases had delayed onset, with development of POI several years after the diagnosis.
We also found a statistically significant difference in the time elapsed to the development of POI based on the stage of pubertal development at the time of the cancer diagnosis, with substantially earlier development of POI in patients diagnosed after puberty compared to patients diagnosed in prepubertal stages. There is published evidence of a lower probability of POI in female patients treated at earlier ages, which is associated with the greater number of primordial follicles.25 This could also explain the delay in the development of POI in younger patients, although the data currently available are not sufficient to confirm this hypothesis.
It is important to highlight that while menstruation and oocytes are intimately related, it is possible for menstruation to occur in anovulatory cycles in association with abnormalities in the hypothalamic-pituitary-ovarian axis. The presence of menstruation after cancer treatment is not indicative of an adequate ovarian reserve and may be misinterpreted in some cases.24 Therefore, it should not be the basis for failing to provide adequate follow-up in patients at risk. In our study, menarche had taken place before the development of POI in 36% of cases, and menstrual cycles continued in 23%.
In our study, we analysed risk factors based on the classification of the Pediatric Interest Network of the Oncofertility Consortium.14
At present, multiple tools are available to establish uniform risk group definitions and assist clinicians in risk stratification. Green et al21 developed the Cyclophosphamide Equivalent Dose (CED) calculator to facilitate universal risk stratification independent of the population (https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/). Clark et al. developed an online tool to predict the risk of ovarian failure after treatment.26 Knowledge and use of these tools by specialists allows the identification girls/adolescents at risk following diagnosis and development of a treatment plan. The retrospective use of these tools in our study indicated that 97% of survivors in the sample were at high risk of POI.
In our study, POI was diagnosed in 19 girls that had not undergone puberty or menarche, of who 47% were not in follow-up by an endocrinologist nor undergoing monitoring or hormone levels due to a lack of suspicion. Introducing the routine use of the tools and guidelines described above would contribute substantially to early diagnosis, which carries significant benefits.
The findings in our study were consistent with the predictions of these tools, as all patients with POI had abnormal levels of FSH and oestradiol 63% (n = 19) had no signs of puberty.
The level of AMH is not recommended as a criterion for diagnosis of POI, as low values may be due to toxicity and to the disease in general. Low values of AMH adolescents or young adults do not preclude the possibility of pregnancy. Furthermore, the interpretation of AMH values in the paediatric population is complicated, as the levels increase throughout childhood until adulthood to then decrease with menopause. Overall, with the exception of a study conducted by Van der Kooi et al.,27 there have been no differences in AMH levels after treatment in prepubertal vs postpubertal patients. Most published studies have also found no differences in the levels of adult survivors of childhood or adolescent cancers compared to the general population.28
The combination of FSH elevation and undetectable or low levels of AMH is associated with POI. This was confirmed in our study in the patients in whom AMH levels had been measured (23%), in whom AMH levels were decreased and FSH levels elevated.
In the follow-up of female cancer survivors, AMH levels have been found to be useful to detect poor ovarian reserve and identify the need for close monitoring.29 Another method used to monitor ovarian reserve in adult women is the transvaginal ultrasound scan. As is the case of AMH, transvaginal sonography is not recommended as a criterion for the definition of POI. Its applicability in childhood and adolescence is limited. In our study, this test had only been performed in 10 of the patients (33%), and the findings were pathological, with fewer than 10 follicles, in 90% of them.
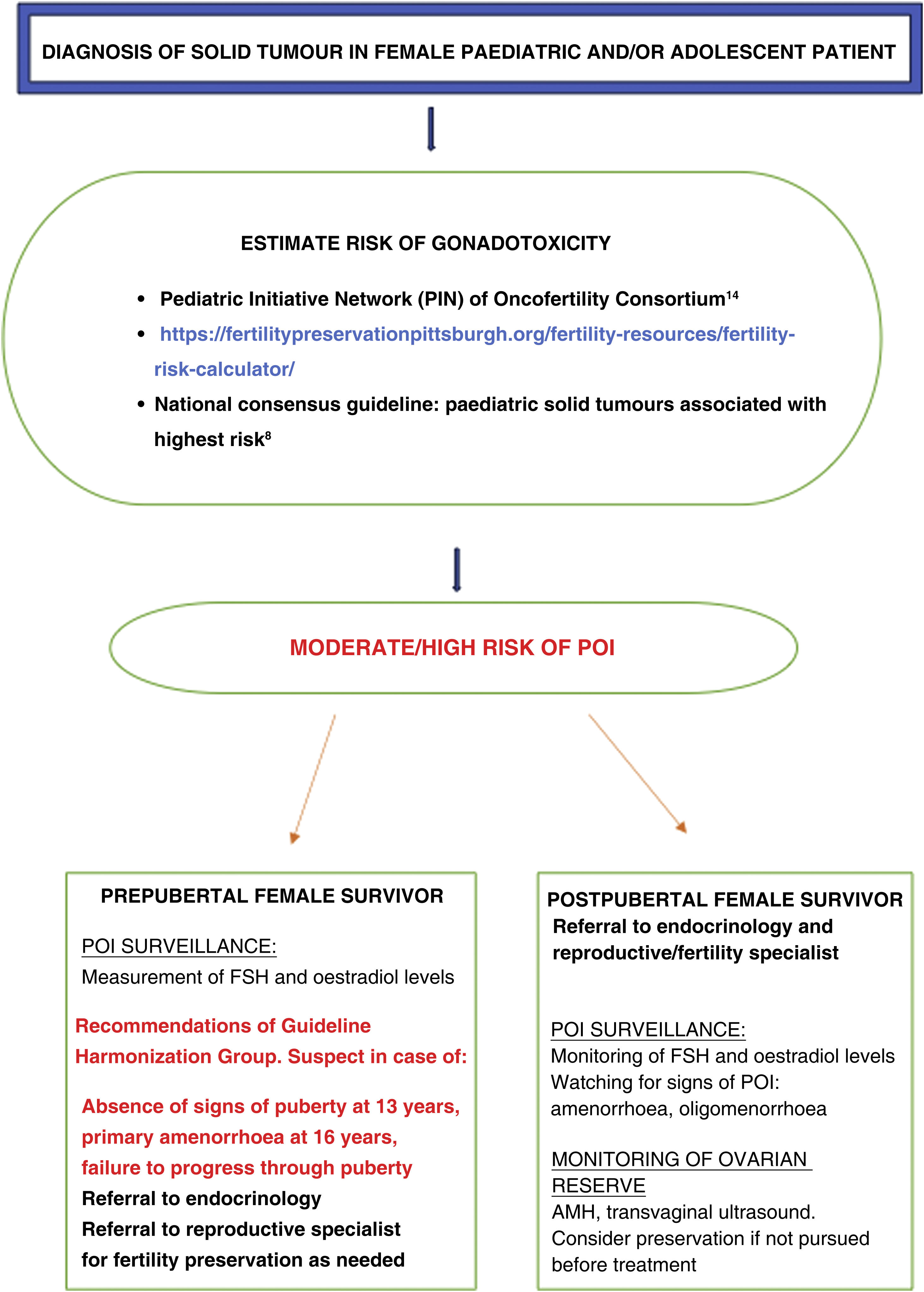
Despite advances in oncofertility, such as the development of guidelines or tools to identify patients at risk,30 as noted above, our study found that the monitoring of the risk of POI was only adequate in 11 patients (37%), even though the entire sample consisted of patients at high risk. In addition, the proportion that had undergone fertility preservation interventions before starting treatment or developing POI was low (17%). Fig. 4 summarises the recommendations and the tools currently available for the follow-up of women at risk of developing POI.
At presence, there is preliminary evidence on the use of anthracyclines and the increased risk of POI,31 while the effect on the gonads of novel treatments like monoclonal antibodies, bevacizumab or tyrosine-kinase inhibitors, among others, is unknown.32 These risk factors were not taken into account in our study and could be analysed in future studies.
ConclusionThe use of the tools currently available to estimate the risk of POI allows the education of patients and/or families regarding potential adverse events and can guide the development of a follow-up plan, including monitoring and referral to the appropriate specialists. There is significant room for improvement in this field in Spain, and improvement could yield substantial health benefits. Chief among them is the possibility of fertility preservation prior to the development of POI in survivors of childhood cancer in which such an intervention was not contemplated or possible before initiation of treatment.33 It would also allow early initiation of hormone replacement therapy, which has been found to decrease the morbidity secondary to cardiovascular disease, decreased bone density and psychological distress. The involvement of endocrinology and gynaecology specialists in the follow-up of these patients is essential.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.