The AEP Immunization Calendar for 2024, with its immunization recommendations for pregnant women, children and adolescents residing in Spain, marks the 25th edition since the first one was introduced in 1995, being annual since 2003, as a vaccination calendar, and since 2023 as immunization schedule due to the inclusion of a monoclonal antibody for the prevention of RSV disease. Novelties for this year include the following:
- •
Tables of systematic immunizations for healthy people and those belonging to risk groups.
- •
Although vaccination recommendations were previously made for pregnant women, they have been now included in the table and a specific section has been created.
- •
Vaccination against pneumococcus is recommended with one of the new expanded valence conjugate vaccines, replacing PCV13.
- •
It is recommended to replace the meningococcus C vaccine at 4 months of age with the MenACWY vaccine, thus leaving the recommended schedule as 1 + 1 + 1 (4 months, 12 months and 12 years, with a catch-up for adolescents up to 18 years).
- •
The intranasal flu vaccine is recommended as the preferred vaccine for people over 2 years of age.
- •
Following the proposals of the WHO, ECDC and CISNS, vaccination against SARS-CoV-2 is now recommended only for people over 6 months of age with risk factors, using vaccines containing the XBB.1 lineage. Vaccination recommendations against covid in pediatrics will be updated periodically on the CAV-AEP website.
The rest of the recommendations from the previous calendar remain unchanged.
El Calendario de Inmunizaciones de la AEP para 2024, con sus recomendaciones de inmunización para embarazadas, niños y adolescentes residentes en España, hace el número 25 desde el primero presentado en 1995, siendo anual desde 2003, como calendario de vacunaciones, y desde 2023 como calendario de inmunizaciones por la inclusión de un anticuerpo monoclonal para la prevención de la enfermedad por VRS. Como novedades de este año, se encuentran las siguientes:
- •
Tabla de inmunizaciones sistemáticas para personas sanas y otra para pertenecientes a grupos de riesgo.
- •
Aunque ya anteriormente se hacían recomendaciones de vacunación en embarazadas, se han añadido a la tabla y se ha creado un apartado específico.
- •
Se recomienda la vacunación frente al neumococo con una de las nuevas vacunas conjugadas de valencia ampliada, en sustitución de VNC13.
- •
Se recomienda la sustitución de la vacuna frente al meningococo C a los 4 meses de edad por la vacuna MenACWY, quedando la pauta recomendada como 1 + 1 + 1 (4 meses, 12 meses y 12 años, manteniendo el rescate en adolescentes hasta los 18 años).
- •
Se recomienda la vacuna intranasal frente a gripe como la preferente en mayores de 2 años.
- •
Siguiendo las propuestas de OMS, ECDC y CISNS, la vacunación frente al SARS-CoV-2 pasa a ser recomendada solo para personas mayores de 6 meses con factores de riesgo, con preparados que contengan el linaje XBB.1. Las recomendaciones de vacunación contra la covid en pediatría se actualizarán periódicamente en la web del CAV-AEP.
Se mantienen el resto de las recomendaciones del calendario anterior.
This document presents the recommendations for immunization for year 2024 of the Advisory Committee on Vaccines (CAV) of the Asociación Española de Pediatría (AEP, Spanish Association of Pediatrics) for individuals aged less than 18 years and pregnant women residing in Spain, including healthy individuals and risk groups (Figs. 1 and 2).
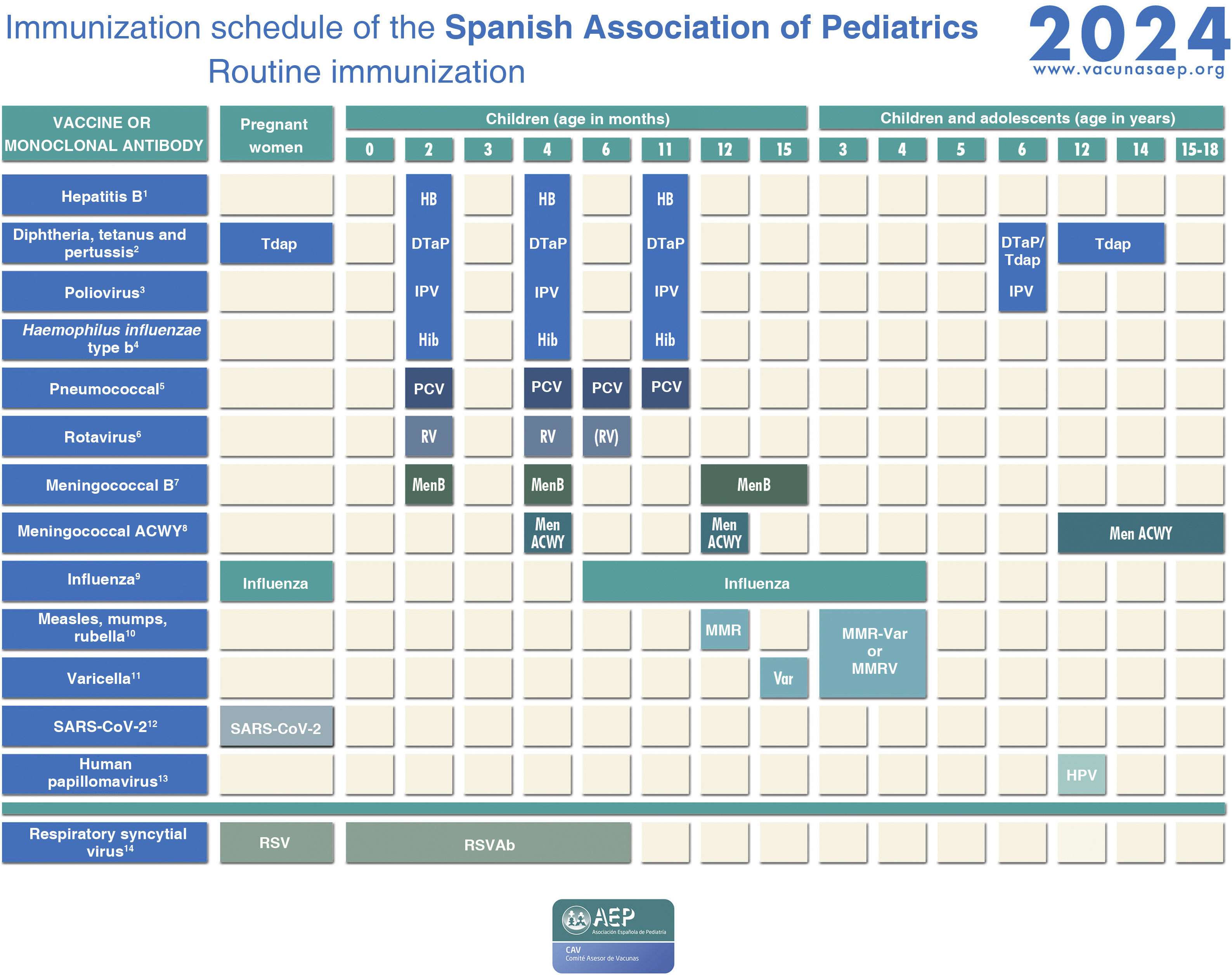
Immunization schedule of the Spanish Association of Pediatrics: 2024 recommendations. Routine immunization.
(1) Hepatitis B vaccine (HB).- Three doses of hexavalent vaccine at 2, 4 and 11 months. Unvaccinated children and adolescents should be given 3 doses of monovalent vaccine on a 0, 1 and 6-month schedule.
(2) Diphtheria, tetanus and acellular pertussis vaccine (DTaP/Tdap).- Five doses: primary vaccination with 2 doses, at 2 and 4 months, and booster at 11 months (third dose) with DTaP-IPV-Hib-HB (hexavalent) vaccine; at 6 years (fourth dose) with the standard load vaccine (DTaP-IPV), preferable to the low diphtheria and pertussis antigen load vaccine (Tdap-IPV), and at 12–14 years (fifth dose) with Tdap. In children previously vaccinated with the 3 + 1 schedule (at 2, 4, 6 and 18 months), it is possible to use the Tdap for the booster at age 6 years, as they do not need additional doses of IPV. Administration of Tdap is recommended in each pregnancy between weeks 27 and 36 of gestation, preferably weeks 27−28. In the case of probable preterm labour, it can be administered from week 20, after performance of the high-resolution foetal ultrasound scan.
(3) Inactivated poliovirus vaccine (IPV).- Four doses: primary vaccination with 2 doses, at 2 and 4 months, and booster doses at 11 months (with hexavalent vaccine) and 6 years (with DTaP-IPV or Tdap-IPV). Children previously vaccinated with the 3 + 1 schedule (at 2, 4, 6 and 18 months), require no additional doses of IPV. Children from countries that use the oral poliovirus vaccine (OPV) who have been vaccinated with 2 or 3 doses of the bivalent OPV vaccine exclusively (it was in April 2016 that the global switch from the bivalent OPV to the trivalent IPV started, as recommended by the WHO) should be given at least 2 doses of IPV at least 6 months apart to guarantee protection against poliovirus type 2.
(4) Haemophilus influenzae type b conjugate vaccine (Hib).- Three doses: primary vaccination at 2 and 4 months and booster dose at 11 months with hexavalent vaccine.
(5) Pneumococcal conjugate vaccine (PCV).- Three or four doses: 2 + 1 series with PCV15 (at 2, 4 and 11 months) or 3 + 1 series with PCV20 (at 2, 4, 6 and 11 months), when available.
(6) Rotavirus vaccine (RV).- Two or three doses of rotavirus vaccine: at 2 and 3–4 months with the monovalent vaccine or at 2, 3 and 4 months or 2, 3–4 and 5–6 months with the pentavalent vaccine. To minimise the already low risk of intussusception, vaccination must start between 6 and 12 weeks of life and be completed by 24 weeks for the monovalent vaccine and 33 weeks for the pentavalent vaccine. Doses must be given at least 4 weeks apart. Both vaccines may be given at the same time as any other vaccine (with the exception of the oral poliovirus vaccine, which is not currently distributed in Spain).
(7) Meningococcal B vaccine (MenB).- 4CMenB. Three doses: start at age 2 months, with a series of 2 doses 2 months apart and a booster starting from age 12 months and at least 6 months after the last dose in the primary series. Administration of the 4CMenB at the same time as other vaccines in the schedule is recommended. However, if either the clinician or the family prefer not to administer it at the same time, it can be given after any interval of time, although this has the drawback of delaying the protection provided by the vaccine. For all other age groups, the indication of vaccination with either vaccine (4CMenB or MenB-fHbp) is determined on a case-by-case basis, always adhering to the minimum age authorised for each vaccine.
(8) Meningococcal ACWY conjugate vaccine (MenACWY).- One dose of conjugate MenACWY-TT at age 4 months and a booster dose at age 12 months. In adolescence (11–13 years), administration of 1 dose of MenACWY is recommended, in addition to catch-up vaccination through age 18 years. In autonomous communities where the MenACWY vaccine is not included in the routine immunization schedule at 4 and 12 months, if parents choose not to administer it, the MenC-TT vaccine funded by the regional government must be administered instead. For all other age groups, the decision to vaccinate must be made on a case-by-case basis.
(9) Influenza vaccine.- recommended in all children aged 6–59 months with administration of an inactivated vaccine via the intramuscular route (some can be administered via deep subcutaneous injection) or, from age 2 years, preferably with the intranasal live attenuated vaccine. A single dose should be given from age 6 months, except in children aged less than 9 years in risk groups, who should be given 2 doses 4 weeks apart if it is the first time they are vaccinated against influenza. The dose is 0.5 mL delivered intramuscularly in the case of the inactivated vaccine and 0.1 mL in each nostril in the case of the attenuated vaccine. Vaccination against influenza and COVID is recommended in pregnant women in any trimester or in the postpartum period within 6 months of birth if not vaccinated during pregnancy. Both vaccines can be given at the same time.
(10) Measles, mumps and rubella vaccine (MMR).- Two doses of MMR vaccine. The first at age 12 months and the second at age 3–4 years. The quadrivalent MMRV vaccine may be administered for the second dose. In susceptible patients outside the specified ages, vaccination with 2 doses of MMR at least 1 month apart is recommended.
(11) Varicella vaccine (Var).- Two doses: the first one at 15 months (although it is possible to administer from age 12 months) and the second at age 3–4 years. The quadrivalent vaccine (MMRV) may be used for the second dose. Susceptible patients outside the specified ages will be vaccinated with 2 doses of monovalent Var vaccine at least 1 month apart, with a recommended 12-week interval between doses in children aged less than 13 years.
(12) SARS-CoV-2 vaccine.- One dose during pregnancy in any trimester. If pregnant women have been vaccinated before or had the infection, the vaccine should be given at least 3 months after the last exposure event. Vaccination in the postpartum period within 6 months of delivery is also indicated if not performed during the pregnancy. The vaccine can be given at the same time as the influenza or Tdap vaccines.
(13) Human papillomavirus vaccine (HPV).- Universal routine vaccination against HPV in children of any sex at age 10–12 years with 2 doses. The vaccines currently available are HPV2 and HPV9. The higher valency vaccine (HPV9) is recommended. Vaccination schedules: 2-dose series (at 0 and 6 months) between 9 and 14 years and 3-dose series (at 0, 1–2 [depending on vaccine] and 6 months) in individuals aged 15 years or older. It can be administered at the same time as the MenC, MenACWY, hepatitis A and B and Tdap vaccines. There are no data for administration with the varicella vaccine, although it should not cause any problems.
(14) Respiratory syncytial virus (RSV).- Pregnant women should receive the RSVPreF vaccine (Abrysvo) between 24 and 36 weeks of gestation, preferably between weeks 32 and 36. The vaccine is not available for the 2023−2024 season. Administration of 1 dose of nirsevimab (an anti-RSV antibody) is recommended in all neonates born during the RSV season (October-March) and infants aged less than 6 months (born between April and September) at the beginning of the season.
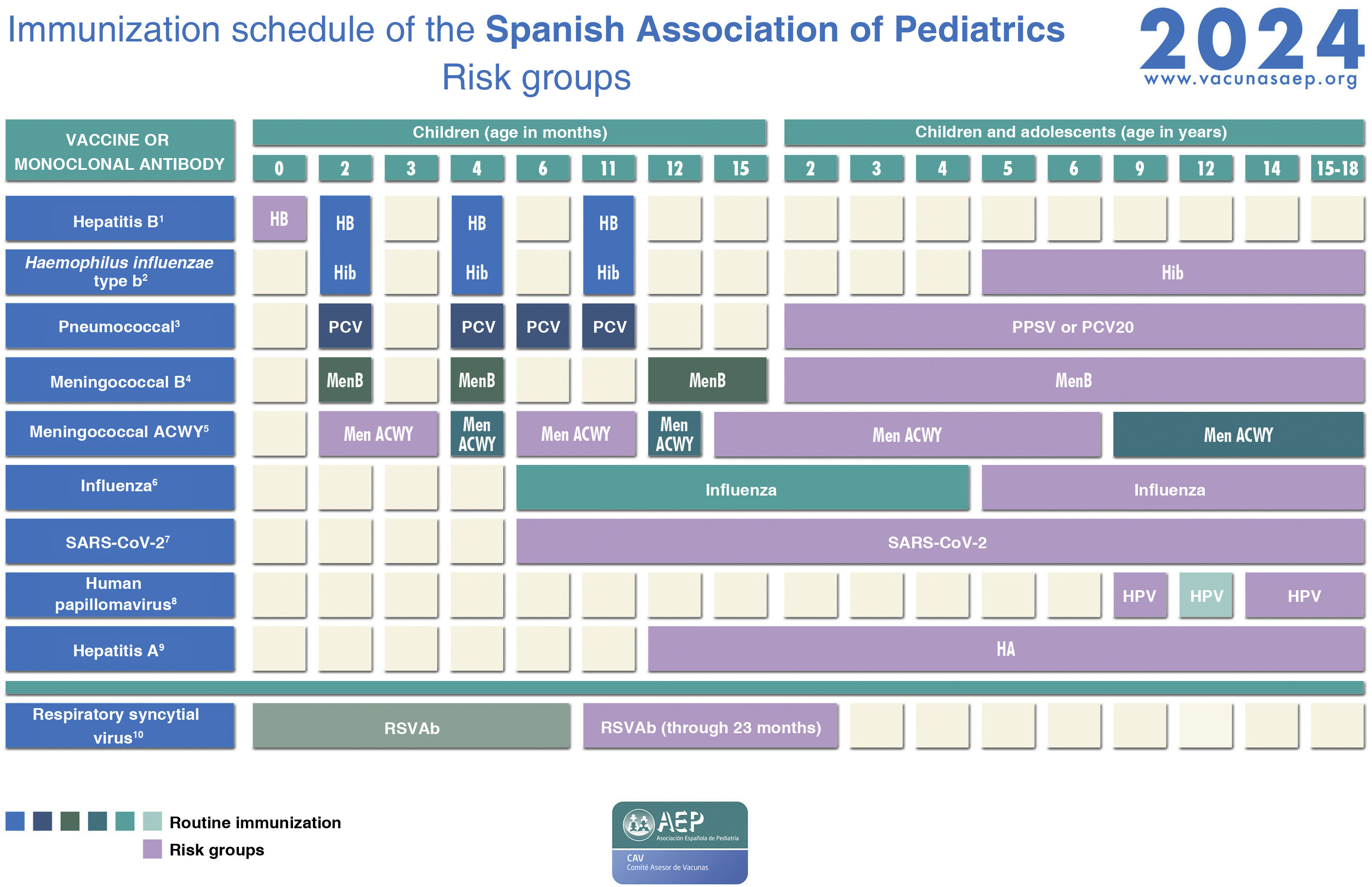
Immunization schedule of the Spanish Association of Pediatrics: 2024 recommendations. Risk groups.
(1) Hepatitis B vaccine (HB).- Children of HBsAg-positive mothers will be given 1 dose of vaccine and 1 dose of hepatitis B immune globulin (HBIG) (0.5 mL) within 12 h of birth. In the case of unknown maternal serologic status, children will receive the vaccine within 12 h of birth, followed by 0.5 mL of HBIG, preferably within 72 h of birth, if maternal HBsAg-positive status is confirmed. Infants vaccinated at birth will adhere to the routine schedule for the first year of life, and thus will receive 4 doses of HB vaccine. There are other risk groups.
(2) Haemophilus influenzae type b vaccine (Hib).- Vaccination in children aged more than 59 months is unnecessary, except in those belonging to risk groups: anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, infection by HIV or history of invasive disease by H influenzae. In in unvaccinated or partially vaccinated children younger than 59 months, vaccinate according to the accelerated or catch-up vaccination schedule of the CAV-AEP.
(3) Pneumococcal polysaccharide vaccine (PPSV).- The 23-valent vaccine (PPSV23) is indicated in children aged more than 2 years fully vaccinated with conjugate vaccine (PCV13 or PCV15) and with any disease increasing the risk of pneumococcal infection (1 or 2 doses, depending on risk factor); it should be given at least 8 weeks apart from the last dose of conjugate vaccine. When we have the 20-valent conjugate vaccine (PCV20), it will replace the dose of PPSV23 in children vaccinated with PCV13 or PCV15. In children who have been fully vaccinated with PCV20 (primary series and booster), administration of PPSV23 is not necessary.
(4) Meningococcal B vaccine (MenB).- 4CMenB. Recommended in risk groups at any age from 1 year (infants under 1 year will be vaccinated according to the routine schedule): anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, haematopoietic stem cell transplant recipients, infection by HIV, prior episode of invasive meningococcal disease (IMD) caused by any serogroup and contacts of an index case of IMD caused by serogroup B in the context of an outbreak. Subsequently, with the exception of children aged less than 2 years or with a history of IMD, 1 dose of MenB should be given one year after completion of the primary series and every 5 years thereafter. In the context of an outbreak of IMD caused by group B, patients in risk groups should be given a booster dose if at least 1 year has elapsed from completion of the primary vaccination series.
(5) Meningococcal ACWY conjugate vaccine(MenACWY).- The MenACWY continues to be particularly recommended for children and adolescents who are going to move to countries where this vaccine is indicated at the corresponding age (Canada, USA, Argentina, Chile, Saudi Arabia, Australia, Andorra, Austria, Belgium, Cyprus, Slovakia, Greece, Ireland, Italy, Malta, Netherlands, United Kingdom, Czech Republic, San Marino, Switzerland) and children in risk groups: anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, haematopoietic stem cell transplant recipients, infection by HIV, prior episode of invasive meningococcal disease (IMD) caused by any serogroup and contacts of an index case of IMD caused by serogroup A, C, W or Y in the context of an outbreak. Primary vaccination at any age with 2 doses at least 2 months apart. If the risk persists, administration of a booster dose is recommended every 3 years in children aged less than 7 years and every 5 years in older children. Travellers to Mecca or the African meningitis belt in the dry season must also be vaccinated with MenACWY.
(6) Influenza vaccine.- Recommended for all risk groups and household contacts from age 6 months. The risk groups relevant to this vaccine can be found in the document outlining the recommendations of the CAV-AEP for the 2023−2024 season.
(7) SARS-CoV-2 vaccine.- According to the recommendations of the Public Health Commission of Spain concerning vaccination against COVID-19 for the 2023−2024 season, vaccination is indicated from age 6 months in individuals with diseases considered a high or very high risk, receiving immunosuppressive treatment or who are household contacts of at-risk individuals, as well as individuals aged 5 years or older living in residential facilities or institutionalised for prolonged periods. Monovalent vaccines against the omicron XBB.1.5 variants should be used: Comirnaty XBB.1.5 (preparations containing 3 µg [age 6 months–4 years], 10 µg [age 5−11 years] or 30 µg [age ≥ 12 years]) or Spikevax XBB.1.5 (available as 0.1 mg/mL multidose vial to deliver 10 doses of 2.5 mL/25 µg [age 6 months–11 years] or 5 doses of 0.5 mL/50 µg [age ≥ 11 years]). Primary vaccination in individuals aged more than 6 months who have had the infection: single dose, at least 3 months after the infection, except in severely immunosuppressed patients, who should receive a second dose at least 3 months after the first one. Primary vaccination in individuals with no history of infection: for those aged 5 years or older, a single dose; for children aged 6 months to 4 years, 3 doses at 0, 3 and 8 weeks of Comirnaty XBB.1.5 3 µg or 2 doses of Spikevax XBB.1.5 (0.25 mL/25 µg) at 0 and 28 days. In children aged 6 months to 4 years who are partially vaccinated, complete the series with one of the new monovalent vaccines. Annual seasonal dose (autumn-winter 2023−2024) in risk groups: single dose, independently of the number of doses received in the past, in those previously vaccinated or with a previous history of SARS-CoV-2 infection at least 3 months after the last dose of vaccine or episode of infection. The risk groups can be consulted in the recommendations published by the Ministry of Health and in the online manual of immunizations of the CAV-AEP.
(8) Human papillomavirus vaccine (VPH).- Vaccination is indicated from age 9 years, always with 3 doses, in immunosuppressed individuals. Consult the Manual of immunizations for other risk groups.
(9). Hepatitis A vaccine (HA).- The pre-exposure and post-exposure risk groups are detailed in our Manual. Infants aged 6–11 months traveling to risk areas can be given the vaccine, but it will not count as a valid dose toward the routine vaccination series, which will have to start over from age 12 months.
(10)Respiratory syncytial virus antibody(RSVAb).- Administration of nirsevimab (anti-VRS antibody) is recommended annually (for 2 seasons) in children aged less than 2 years with underlying disease increasing the risk of severe RSV infection, preferably just before the usual start of the RSV season (October).
The official immunization schedules of the Interterritorial Council of the National Health System of Spain (ICNHS) and the regional governments of the autonomous communities are increasingly approximating the schedule proposed by the CAV-AEP, introducing novel drugs for the prevention of infectious diseases, and Spain is, for instance, the first country to introduce a monoclonal antibody for routine administration (nirsevimab).
We highlight the importance of public health services and primary care paediatric teams in maintaining the high vaccination coverage in Spain, as well as the need for a single immunization schedule to prevent inequities. We collaborate in actions with different governmental agencies, but also advocate for the inclusion of expert paediatricians in the committees that make decisions regarding immunization policy. We propose the implementation of alternative funding strategies for immunizations that are not currently included in the routine schedule, and we ask for greater social commitment from pharmaceutical companies to facilitate access to preventive products to the population.
Table 1 summarises the sources from which, in adherence with current standards for the critical review of the scientific literature, we obtained the information required to develop the recommendations for 2024.
Bibliographic sources and literature search strategies (CAV-AEP).
| • TripDatabase: Advanced search: (disease) (vaccine) (vaccination) |
| • Cochrane Library: Disease AND vaccine |
| • MEDLINE/Pubmed: (“disease/microorganism” [MeSH Terms]) AND (“vaccine” [MeSH Terms] OR “vaccination” [MeSH Terms]). Filters activated: childbirth-18 years, human (Sort by: Best Match) |
| • EMBASE: “disease”/exp AND “vaccine”/exp |
| • Official websites of the Ministry of Health of Spain and the Instituto de Salud Carlos III (ISCIII) |
| • Websites of medicines regulatory authorities: Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) and European Medicines Agency (EMA) |
| • CAV-AEP. Summaries of product characteristics |
| • Government agencies or international advisory bodies involved in vaccine policy: ACIP (EE UU.), JCVI (United Kingdom), STIKO (Germany), Public Health Agency of Canada, Australian Department of Health |
| • Communications and presentations in national and international congresses |
| • Primary sources (textbooks, references of articles selected in the search) |
| • Data obtained directly from authors (unpublished) |
| • Publications not indexed in databases |
| • Information obtained from the pharmaceutical industry |
2024 recommendation. Pertussis: dose of tetanus and reduced diphtheria and acellular pertussis toxoid vaccine (Tdap) in each pregnancy from 27 weeks of gestation, preferably on week 27−28. Influenza: vaccination during the flu season in any trimester of pregnancy or in the postpartum period if not vaccinated during pregnancy. SARS-CoV-2: vaccination in any trimester or booster dose, as applicable. Respiratory syncytial virus (RSV): when indicated as part of a public health strategy, administer a dose between weeks 24 and 36 of gestation, preferably between weeks 32 and 36.
Vaccination with Tdap during pregnancy protects newborns and infants before the start of routine immunization.1 Recommended in each pregnancy between 27 and 36 weeks of gestation, preferably on weeks 27−28. If preterm delivery is likely, it is possible to administer it from week 20, after performance of a high-resolution foetal ultrasound.
Pregnant women are at increased risk of complications and hospitalization due to influenza or SARS-CoV-2 infection, in addition to adverse perinatal events such as preterm birth and low birth weight.2 Vaccination against both is recommended and can be performed in any trimester of the pregnancy and up to 6 months postpartum if vaccination was nor performed during the pregnancy. Both vaccines can be administered at the same time. The SARS-CoV-2 vaccine should be administered independently of the number of previously received doses and at least 3 months apart from the last dose or infection.3,4
Recently, a vaccine against RSV (RSV prefusion F protein-based vaccine [RSVPreF]) was authorised for use in pregnant women aimed at the passive immunization of their future offspring. The efficacy of the vaccine in preventing severe lower respiratory tract infections (LRTIs) is of 81.8% in the first 90 days of life and 69.4% at 6 months post birth.5 The European Medicines Agency (EMA) has approved its use between 24 and 36 weeks (the CAV-AEP considers administration between 32 and 36 weeks preferable). When this vaccine is available, the competent public health authorities will determine the best strategy (nirsevimab and/or RSVPreF) for the prevention of RSV disease in newborns and infants aged less than 6 months, taking into account multiple factors that could have an impact on its success, in terms of both effectiveness and vaccination coverage.
Vaccination with hexavalent vaccine (DTaP-HB-Hib-IPV)2024 recommendation: 2 + 1 series with hexavalent diphtheria, tetanus and acellular pertussis (DTaP), hepatitis B (HB) Haemophilus influenzae type B (Hib) and inactivated poliovirus (IPV) (DTaP-HB-Hib-IPV) vaccine (at 2, 4 and 11 months); DTaP-IPV or Tdap-IPV at 6 years and Tdap at 12–14 years. In children and adolescents not vaccinated against HB, administration at any age of 3 doses of monovalent vaccine (or combined with hepatitis A vaccine, if indicated) at 0, 1 and 6 months.
If the vaccination coverage is high, vaccination with hexavalent vaccine is very effective with a 2 + 1 series (with an interval of at least 2 months between primary series doses and 6 months between the primary series and the booster dose), a schedule introduced in Spain in 2016−17 that requires administration of a fourth dose of IPV; in 2022, vaccination with DTaP-IPV at age 6 years was introduced (although Tdap-IPV may be used if DTaP-IPV is not available or the child is aged more than 7 years).
With the administration of Tdap in adolescence, patients receive a fifth dose of tetanus and diphtheria vaccine, thus completing vaccination against tetanus and receiving a booster against pertussis.
Spain is a low-endemic country for HB in which vertical transmission is rare and paediatric infection infrequent. The full vaccination series induces a lasting seroprotective response (antiHB antibodies > 10 mIU/mL) in more than 95% of vaccinated individuals. The response is lower in immunosuppressed individuals and those treated with haemodialysis, who should be given and adjuvanted vaccine (4 doses at 0, 1, 2 and 6 months) if their age is greater than 15 years or a larger dose of standard vaccine if their age is less than 15 years. The lesser response in patients with coeliac disease calls for assessment of the serological response after vaccination.6
Vaccination against pneumococcal disease2024 recommendation: vaccination against pneumococcal disease in children aged less than 5 years and at any age in risk groups. Either of the following schedules is recommended for routine vaccination of healthy infants: 2 + 1 doses (2, 4 and 11 months) of 15-valent pneumococcal conjugate vaccine (PCV15) or 3 + 1 doses (2, 4, 6 and 11 months) of 20-valent pneumococcal conjugate vaccine (PCV20), when available.
During the SARS-CoV-2 pandemic, there was a marked decrease in the incidence of invasive pneumococcal disease (IPD) as well as non-invasive disease7 as a result of the implemented containment measures and the associated decrease in the circulation of respiratory viruses (RSV, metapneumovirus, influenza viruses). However, once the containment measures were lifted, the incidence of viral respiratory tract infections surged, as did pneumococcal infections,8 whose incidence quickly reached or even exceeded prepandemic levels, especially in young children.9,10
In the prepandemic period, the most prevalent serotypes involved in paediatric IPD were 24F, 8, 3 and 33F,11 which are also the most prevalent now, as the pandemic seemingly did not affect the distribution of circulating serotypes,9 although there has been a concerning increase in the frequency of penicillin resistance in serotypes 11A, 24F and 23B.12
The CAV-AEP considered that higher-valency vaccines against pneumococcal disease (PCV15, PCV20) should replace the PCV13 in child immunization schedules.
Vaccination against rotavirus2024 recommendation: the rotavirus vaccine should be included in the routine immunization schedule for all infants.
Rotaviruses (RVs) are the leading cause of acute gastroenteritis in infants worldwide. No risk groups have been identified, with the exception of preterm infants, who are particularly vulnerable to RV infections and at risk of more severe forms of disease compared to their full-term peers.13 Hygiene and disinfection measures have a limited impact on the control of RV, so vaccination is the best prevention strategy currently available.14
The World Health Organization (WHO) and different scientific societies, such as the AEP, recommend routine vaccination against RV. Currently, 116 countries include vaccination against RV in their immunization programmes, 25 of which are in Europe. In Spain, it has been included in the routine immunization schedules of the autonomous communities of Castilla y León (at 2, 3 and 4 months) and Galicia (at 2 and 4 months). There is no evidence of replacement by non-vaccine serotypes.15
The Sociedad Española de Neonatología (Spanish Society of Neonatology) and the CAV-AEP recommend vaccination of preterm infants, even if they are hospitalized. The safety and efficacy data in preterm infants are similar to those in term infants.
Vaccination against meningococcal disease2024 recommendation: routine vaccination against group B meningococcus (MenB vaccine) at age 2 months with a 2 + 1 serious and against groups A, C, W and Y (MenACWY vaccine, at 4 months, 12 months and 12 years and for catchup vaccination in adolescents aged 13 and 18 years). For all other age groups, the decision to vaccinate will be made on a case-by-case basis.
In Spain, invasive meningococcal disease (IMD) is endemic and is associated with groups B, C, W, and Y. The incidence is highest in the first 2 years of life, with a second peak in adolescence. Group B is currently the most prevalent in every age group.16
The global increase in the incidence of IMD caused by groups W and Y in the past decade17 led some countries to replace the meningococcal C (MenC) vaccine by MenACWY in adolescence (for direct protection and possibly for herd immunity), while other countries introduced the administration of this vaccine to infants aged 2–24 months (for direct protection), and others implemented combined strategies.
In Spain, following the important reduction in incidence during the SARS-CoV-2 pandemic, there has been an increase in IMD caused by groups W and Y in the 2022–2023 season, although the incidence has yet to reach prepandemic levels. Compared to the previous season, the number of cases and rates of infection by both group W and Y have tripled.18,19
Given the unpredictable epidemiological trends of disease and the fact that the pandemic interrupted catch-up vaccination in the population aged 13–18 years, making it difficult to reach a vaccination coverage that could offer indirect protection, the direct protection strategy for infants still applies.
As regards the schedule of vaccination, and considering the published results of the study assessing the 1 + 1 series (at 3–12 months) with the combined MenACWY and tetanus toxoid (MenACWY-TT) vaccine,20 we recommend replacing the dose of MenC given at 4 and 12 months by MenACWY, as previously implemented in Galicia, maintaining the vaccine dose at 12 years and catch-up vaccination through age 18 years.
At the individual level, children aged 1–12 years may benefit from these vaccines, achieving greater protection against IMD.
In respect of the multicomponent meningococcal B vaccine (4CMenB), the data on the effectiveness study conducted in infants in Spain21 found a vaccine effectiveness (VE) of 71% for the full series, of 64% for at least 1 dose of vaccine and of 82% for protection against IMD by non-vaccine serotypes of groups other than B. The CAV-AEP applauds the decision made by the Public Health Committee (11/2022) to include routine vaccination against MenB, which was introduced in December 2022 in the official lifespan immunization schedule, and its implementation in every autonomous community in Spain from 2023 with a 2 + 1 series starting at 2 months in pursuit of the optimal protection of infants.
The meningococcal serogroup B-factor H binding protein vaccine (MenB-fHbp) and the 4CMenB have proven effective in protecting against MenB when administered during adolescence.22 In South Australia, the routine vaccination in adolescents achieves a VE of 78.5%, with an added effectiveness of 33.2% in the protection against gonorrhea.23
Few studies have assessed the need of a booster dose in healthy adolescents without risk factors vaccinated with a full series of 4CMenB during childhood, and the results have not been conclusive, so at present we do not recommend routine administration of a booster dose.
However, epidemiological trends are unpredictable and these recommendations may need to be updated. The progressive increase in the incidence of MenB after the pandemic has led to outbreaks in adolescents in the United Kingdom24 and France25 that required the implementation of regional vaccination campaigns.
The CAV-AEP considers that, at the individual level, children and adolescents who have not been previously vaccinated against MenB can be vaccinated with any of the vaccines available for their age.
Vaccination against influenza2024 recommendation: routine yearly vaccination of children aged 6–59 months, children and adolescents in risk groups and household contacts of individuals in risk groups or infants aged less than 6 months. The intranasal vaccine is preferred in children aged more than 2 years.
Influenza is associated with a high burden of disease (30%–40%) in children, who also play a key role in the transmission of the virus to the rest of the community, especially vulnerable groups.26 Since 2012, the WHO recommends annual vaccination of children aged less than 5 years.
For the 2023−2024 season, the ICNHS recommends universal vaccination against influenza in children aged 6–59 months,4 a strategy that will be implemented for the first time in the entire Spanish territory.
There are 5 quadrivalent vaccines that are safe and effective in children, four inactivated vaccines and one attenuated intranasal vaccine (of which the latter is preferred from age 2 years).
Vaccination against measles, mumps and rubella (MMR)2024 recommendation: 1st dose at age 12 months using MMR vaccine, 2nd dose at age 3–4 years with the combined measles, mumps, rubella and varicella vaccine (MMRV).
The WHO recommends a vaccination coverage greater than 95% for at least 1 dose of vaccine, a target that has been achieved in many European countries. The cases of measles reported by the European Centre for Disease Prevention and Control (ECDC)27 amount to an incidence of 0.3 cases per million inhabitants, well below the prepandemic incidence, although higher compared to 2021. Spain, a country free of indigenous measles transmission, reported 1 case of measles and 43 of mumps.
A first dose of MMR administered erroneously or for other reasons between 11 and 12 months is considered valid, as there is evidence that maternal antibodies are present in lower titres and are metabolised faster in the offspring of vaccinated women, which decreases the potential inhibition of the response to the vaccine.28
For epidemiological reasons, the MMR can be administered from age 6 months, but it will still be necessary to administer 2 doses from age 12 months at least 4 weeks apart.
We continue recommending separate administration of the MMR and varicella vaccines for the first dose of the series in children aged less than 2 years due to the increased risk of febrile seizures.
Vaccination against varicella2024 recommendation: routine vaccination with 2 doses (at 15 months and 3–4 years, the MMRV can be administered for the second dose). In unvaccinated children and adolescents without a history of varicella, catch-up vaccination with two doses is recommended.
The vaccines currently available are two monovalent vaccines and the MMRV, all of which are live attenuated vaccines and are highly effective (VE, 92%–97%).
Vaccination against varicella was included in the routine immunization schedule of every autonomous community in 2016. The MMRV is administered for the second dose in 10 of them.
The incidence of herpes zoster is lower in children vaccinated against varicella compared to children who had the natural infection.29
Vaccination against SARS-CoV-22024 recommendation: vaccination with the new vaccines adapted to the omicron XBB.1 variant in children aged more than 6 months at risk of developing severe COVID.
In May 2023, the WHO published a statement informing it no longer considered COVID-19 a public health emergency of international concern,30 which entailed a change in preventive policy against SARS-CoV-2, focusing resources on the most vulnerable groups. In the past year, due to the spread of different omicron variants and high vaccination coverages, there has been a substantial decrease in COVID-related hospital admissions and mortality (a trend also observed in children).31 The CAV-AEP, in agreement with the WHO and the ICNHS,4 recommends vaccination of children aged more than 6 months with risk factors for severe COVID.
International organizations and regulatory agencies (ECDC, United States Centers for Disease Control and Prevention [CDC], EMA and United States Food and Drug Administration [FDA]), in adherence to the recommendations of the Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) of the WHO, have chosen to abandon compositions that include the original Wuhan lineage and propose the use of monovalent vaccines against the XBB.1 omicron lineage (XBB.1.5 and XBB.1.16), adopting a strategy similar to the one used against influenza, by which only individuals in the groups included in Table 2 are considered eligible for routine annual vaccination against SARS-CoV-2.
Diseases associated with the risk of severe COVID in children and adolescents.
| • Chronic cardiovascular, neurologic, neuromuscular or respiratory diseases, including bronchopulmonary dysplasia, cystic fibrosis and severe asthma |
| • Metabolic diseases, including diabetes mellitus and Cushing syndrome |
| • Chronic kidney disease and nephrotic syndrome |
| • Chronic liver disease |
| • Malignant solid and blood tumours |
| • Haemoglobinopathies, anaemias or haemophilia, other coagulopathies and chronic haemorrhagic diseases, including recipients of blood products and multiple transfusions. |
| • Asplenia or severe splenic dysfunction |
| • Cerebrospinal fluid fistula and cochlear implant (current carrier or awaiting placement) |
| • Coeliac disease |
| • Chronic inflammatory disease |
| • Diseases and syndromes manifesting with cognitive impairment: Down syndrome, dementia and other |
| • Morbid obesity (BMI ≥ 35 in adolescents or BMI z ≥ 3 SD in children) |
| • Pregnant adolescents |
| • Immunosuppression (including primary immunodeficiency, immunosuppression caused by HIV infection or pharmaceuticals, and in transplant recipients or patients with complement deficiencies. The following are considered to correspond to a severe level of immunosuppression: |
| - haematopoietic stem cell or solid organ transplant recipients |
| - advanced chronic kidney disease |
| - HIV infection with CD4 < 200 cells/mm3 |
| - some severe primary immunodeficiencies |
| - recipients of certain immunosuppressive treatments (see list in the recommendations of the Interterritorial Council of the NHS for vaccination against influenza and COVID for the 2023−2024 season) |
| • Individuals aged 5 years or older living in residential or locked facilities or institutionalized for prolonged periods of time. |
| • It is also possible to include household contacts of individuals with the previous factors or individuals at risk aged 60 years or older |
Adapted from: Advisory Committee on Vaccines of the AEP (CAV-AEP). Virus SARS-CoV-2. Manual de inmunizaciones en línea de la AEP [Internet]. Madrid: AEP; Dic/2023 and the Recomendaciones de vacunación frente a gripe y COVID-19 en la temporada 2023−2024 of the Interterritorial Council of the National Health System of Spain [online].
Although vaccinating healthy children is no longer a global priority, the roadmap for prioritizing uses of COVID-19 vaccines of the Strategic Advisory Group of Experts on Immunization (SAGE) of the WHO (WHO-SAGE) of 03/2023 contemplates the adaptation of SARS-CoV-2 vaccination policy based on the priorities of national health care systems.32
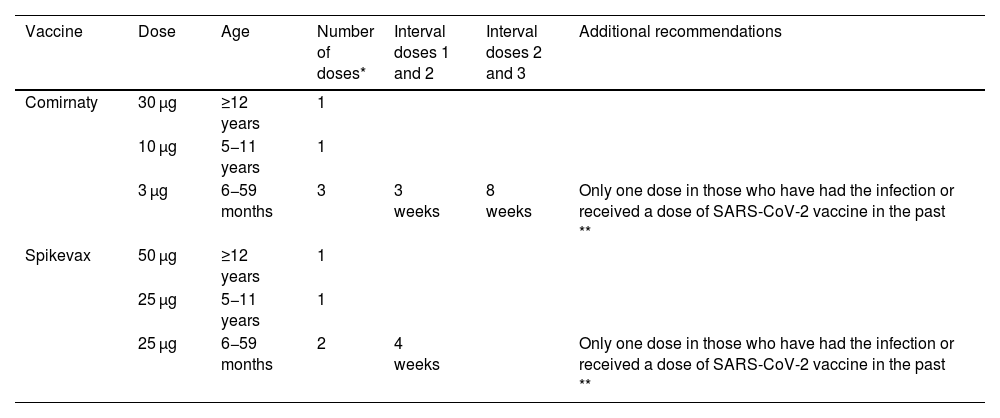
At the time of this writing, the EMA had authorised 2 new monovalent vaccines: Comirnaty XBB1.5 (available in doses of 3, 10 and 30 µg) and Spikevax XBB.1.5 (at doses of 25 or 50 µg) with the schedule presented in Table 3, which is also recommended by the ICNHS. In addition, individuals aged more than 6 months with severe immunosuppression (haematopoietic stem cell or solid organ transplant recipients, patients with advanced chronic kidney disease, HIV + with a CD4 count < 200 cells/mm3, severe primary immunodeficiency or recipients of treatments that cause significant immunosuppression) require an additional dose, to be given at least 3 months apart from the previous one.
Recommendations for vaccination against SARS-CoV-2 (XBB.1.5) of children under 18 years who belong to risk groups—2023-2024.
| Vaccine | Dose | Age | Number of doses* | Interval doses 1 and 2 | Interval doses 2 and 3 | Additional recommendations |
|---|---|---|---|---|---|---|
| Comirnaty | 30 µg | ≥12 years | 1 | |||
| 10 µg | 5−11 years | 1 | ||||
| 3 µg | 6−59 months | 3 | 3 weeks | 8 weeks | Only one dose in those who have had the infection or received a dose of SARS-CoV-2 vaccine in the past ** | |
| Spikevax | 50 µg | ≥12 years | 1 | |||
| 25 µg | 5−11 years | 1 | ||||
| 25 µg | 6−59 months | 2 | 4 weeks | Only one dose in those who have had the infection or received a dose of SARS-CoV-2 vaccine in the past ** |
Adapted from: Advisory Committee on Vaccines of the AEP (CAV-AEP). Virus SARS-CoV-2. Manual de inmunizaciones en línea de la AEP [Internet]. Madrid: AEP; Dic/2023 and the Recomendaciones de vacunación frente a gripe y COVID-19 en la temporada 2023−2024 of the Interterritorial Council of the National Health System of Spain [online].
Individuals with severe immunosuppression aged 6 or more months (see Table 2) will require an additional dose 12 weeks after the last received dose.
2024 recommendation: routine vaccination at age 10–12 years. Vaccination with 9-valent vaccine (HPV9) is recommended.
The recommended age for vaccination is 10–12 years, prior to sexual debut, with the aim of maximizing benefits and vaccination coverage. It is also important to maintain catch-up vaccination and vaccination of individuals in risk groups.
Most HPV infections are transient and resolve within 12–24 months, but in 3%–10% of cases, the infection persists and carries a risk of cervical cancer as well as other types of malignant disease, such as anal or head and neck cancers.
There current evidence shows that vaccination protects against persistent infection, genital warts, premalignant cervical and anal lesions, cervical cancer in women33 and anal cancer in men.34
Multiple studies35,36 have demonstrated the excellent safety profile of HPV vaccines in different age groups.
Prevention of infection by respiratory syncytial virus (RSV)2024 recommendation: routine administration of one dose of nirsevimab to all infants under 6 months and one annual dose in at-risk children aged less than 2 years.
Infection by RSV is associated with a high morbidity and mortality and is a risk factor for the development of bronchial hyperresponsiveness and asthma. Preterm infants, low birth weight infants and patients with immunodeficiencies or chronic disease are considered risk groups.
The elucidation of the refusion and postfusion configurations of the F protein has allowed the development of vaccines (RSVPreF, already discussed in the section on vaccination during pregnancy) and monoclonal antibodies (nirsevimab), both of which have been approved by the EMA.
Nirsevimab is a potent recombinant human monoclonal antibody with a prolonged half-life, that confers protection during the entire RSV season with a single dose. It has been found efficacious in reducing infection requiring medical care in healthy as well as preterm infants (gestational age [GA] ≥ 29 weeks)37–39 and, extrapolating pharmacokinetic data, infants with chronic lung disease, congenital heart disease or born extremely preterm (GA < 29 weeks),40 with a VE of 79.0% for the prevention of LRTI, 80.6% for the prevention of requiring medical care, and 86.2% for the prevention of intensive care unit (ICU) admission, conferring protection against both the A and B subtypes of RSV, with no evidence of a shift in respiratory disease in the second year and a good safety profile. The preliminary findings of the HARMONIE phase IIIb trial (presented at the 2023 Annual Meeting of the European Society for Paediatric Infectious Diseases [ESPID]), conducted in nearly 250 centres in France, Germany and United Kingdom during the 2022–2023 RSV season in a real-world clinical trial setting found an 83.21% reduction in hospitalization due to RSV, a 75.71% reduction in admissions due to severe RSV infection and a 58.04% reduction in hospitalization due to IRTI of any aetiology, with a favourable safety profile and consistent with the findings of pivotal studies. With these outcomes, it is reasonable to infer that the health care and economic burden could be significantly reduced if nirsevimab were administered to all infants.
In consequence, the CAV-AEP recommends administration of nirsevimab to all infants under 6 months and annual administration (total of 2 seasons) to children under 2 years with underlying disease associated with an increased risk of severe infection by.
FundingThe development of these recommendations (analysis of the published data, debate, consensus and publication) has not been supported by any funding source outside of the logistic support provided by the AEP.
Conflicts of interest (last 5 years)FJAG has collaborated in educational activities funded by Alter, AstraZeneca, GlaxoSmithKline, MSD, Pfizer and Sanofi and as a consultant in de GlaxoSmithKline, MSD, Pfizer and Sanofi advisory boards.
AIA has collaborated in educational activities funded by AstraZeneca and GlaxoSmithKline, MSD and Pfizer and as a consultant in GlaxoSmithKline and Pfizer advisory boards. He has also received funding from GlaxoSmithKline, MSD and Pfizer to attend domestic educational activities.
JAA has collaborated in educational activities funded by AstraZeneca, GlaxoSmithKline, MSD, Pfizer, Sanofi and Seqirus, as a researcher in clinical trials for GlaxoSmithKline and Sanofi and as a consultant in AstraZeneca, GlaxoSmithKline, MSD, Pfizer and Sanofi advisory boards.
MGS has collaborated in educational activities funded by Astra, GlaxoSmithKline, MSD, Pfizer and Sanofi, as a researcher in clinical trials for GlaxoSmithKline, Janssen, MSD, Pfizer and Sanofi and as a consultant in GlaxoSmithKline, Novartis and Pfizer advisory boards.
EGL has received funding to attend domestic educational activities and has participated in educational activities funded by GlaxoSmithKline, MSD, Pfizer and Sanofi, as a researcher in clinical trials for e GlaxoSmithKline and MSD, and as a consultant in a GlaxoSmithKline. advisory board.
AMM has collaborated as a researcher without remuneration in a study sponsored by MSD in 2019−20. In the past 5 years, he has not received fees or any form of direct funding from the pharmaceutical industry.
MLNG has collaborated in educational activities funded by Gilead, GlaxoSmithKline, Janssen, MSD, Pfizer and ViiV, as a consultant in Abbott, AstraZeneca, Novartis and ViiV advisory boards and as a researcher in clinical trials sponsored by GlaxoSmithKline, Pfizer, Roche and Sanofi.
VPS as received funding from MSD, Pfizer and Sanofi to attend educational activities in Spain and abroad, has collaborated in educational activities funded by AstraZeneca, GlaxoSmithKline, MSD, Pfizer and Sanofi and as a consultant in GlaxoSmithKline, Pfizer and Sanofi advisory boards.
IRC has collaborated in educational activities funded by GlaxoSmithKline, MSD, Pfizer and Sanofi, as a researcher in vaccine clinical trials for Abbot, AstraZeneca, Enanta, Gilead, GlaxoSmithKline, HIPRA, Janssen, Medimmune, Merck, Moderna, MSD, Novavax, Pfizer, Reviral, Roche, Sanofi and Seqirus and as a consultant in GlaxoSmithKline, MSD, Pfizer and Sanofi advisory boards.
JRC has collaborated in educational activities funded by GlaxoSmithKline, MSD, Pfizer and Sanofi and as a researcher in clinical trials for GlaxoSmithKline and Pfizer.
PSM has collaborated in educational activities funded by Astra-Zeneca, GlaxoSmithKline and MSD, as a researcher in clinical trials for Sanofi and as a consultant in GlaxoSmithKline and Sanofi advisory boards. He has also received funding from GlaxoSmithKline, MSD and Pfizer to attend educational activities in Spain and abroad, and received grants sponsored by GlaxoSmithKline. GlaxoSmithKline.
We thank Javier Arístegui, María José Cilleruelo Ortega, José María Corretger, Nuria García Sánchez, Ángel Hernández Merino, Manuel Merino Moína and Luis Ortigosa for their in-house guidance in the development and writing of these recommendations.





![Immunization schedule of the Spanish Association of Pediatrics: 2024 recommendations. Routine immunization. (1) Hepatitis B vaccine (HB).- Three doses of hexavalent vaccine at 2, 4 and 11 months. Unvaccinated children and adolescents should be given 3 doses of monovalent vaccine on a 0, 1 and 6-month schedule. (2) Diphtheria, tetanus and acellular pertussis vaccine (DTaP/Tdap).- Five doses: primary vaccination with 2 doses, at 2 and 4 months, and booster at 11 months (third dose) with DTaP-IPV-Hib-HB (hexavalent) vaccine; at 6 years (fourth dose) with the standard load vaccine (DTaP-IPV), preferable to the low diphtheria and pertussis antigen load vaccine (Tdap-IPV), and at 12–14 years (fifth dose) with Tdap. In children previously vaccinated with the 3 + 1 schedule (at 2, 4, 6 and 18 months), it is possible to use the Tdap for the booster at age 6 years, as they do not need additional doses of IPV. Administration of Tdap is recommended in each pregnancy between weeks 27 and 36 of gestation, preferably weeks 27−28. In the case of probable preterm labour, it can be administered from week 20, after performance of the high-resolution foetal ultrasound scan. (3) Inactivated poliovirus vaccine (IPV).- Four doses: primary vaccination with 2 doses, at 2 and 4 months, and booster doses at 11 months (with hexavalent vaccine) and 6 years (with DTaP-IPV or Tdap-IPV). Children previously vaccinated with the 3 + 1 schedule (at 2, 4, 6 and 18 months), require no additional doses of IPV. Children from countries that use the oral poliovirus vaccine (OPV) who have been vaccinated with 2 or 3 doses of the bivalent OPV vaccine exclusively (it was in April 2016 that the global switch from the bivalent OPV to the trivalent IPV started, as recommended by the WHO) should be given at least 2 doses of IPV at least 6 months apart to guarantee protection against poliovirus type 2. (4) Haemophilus influenzae type b conjugate vaccine (Hib).- Three doses: primary vaccination at 2 and 4 months and booster dose at 11 months with hexavalent vaccine. (5) Pneumococcal conjugate vaccine (PCV).- Three or four doses: 2 + 1 series with PCV15 (at 2, 4 and 11 months) or 3 + 1 series with PCV20 (at 2, 4, 6 and 11 months), when available. (6) Rotavirus vaccine (RV).- Two or three doses of rotavirus vaccine: at 2 and 3–4 months with the monovalent vaccine or at 2, 3 and 4 months or 2, 3–4 and 5–6 months with the pentavalent vaccine. To minimise the already low risk of intussusception, vaccination must start between 6 and 12 weeks of life and be completed by 24 weeks for the monovalent vaccine and 33 weeks for the pentavalent vaccine. Doses must be given at least 4 weeks apart. Both vaccines may be given at the same time as any other vaccine (with the exception of the oral poliovirus vaccine, which is not currently distributed in Spain). (7) Meningococcal B vaccine (MenB).- 4CMenB. Three doses: start at age 2 months, with a series of 2 doses 2 months apart and a booster starting from age 12 months and at least 6 months after the last dose in the primary series. Administration of the 4CMenB at the same time as other vaccines in the schedule is recommended. However, if either the clinician or the family prefer not to administer it at the same time, it can be given after any interval of time, although this has the drawback of delaying the protection provided by the vaccine. For all other age groups, the indication of vaccination with either vaccine (4CMenB or MenB-fHbp) is determined on a case-by-case basis, always adhering to the minimum age authorised for each vaccine. (8) Meningococcal ACWY conjugate vaccine (MenACWY).- One dose of conjugate MenACWY-TT at age 4 months and a booster dose at age 12 months. In adolescence (11–13 years), administration of 1 dose of MenACWY is recommended, in addition to catch-up vaccination through age 18 years. In autonomous communities where the MenACWY vaccine is not included in the routine immunization schedule at 4 and 12 months, if parents choose not to administer it, the MenC-TT vaccine funded by the regional government must be administered instead. For all other age groups, the decision to vaccinate must be made on a case-by-case basis. (9) Influenza vaccine.- recommended in all children aged 6–59 months with administration of an inactivated vaccine via the intramuscular route (some can be administered via deep subcutaneous injection) or, from age 2 years, preferably with the intranasal live attenuated vaccine. A single dose should be given from age 6 months, except in children aged less than 9 years in risk groups, who should be given 2 doses 4 weeks apart if it is the first time they are vaccinated against influenza. The dose is 0.5 mL delivered intramuscularly in the case of the inactivated vaccine and 0.1 mL in each nostril in the case of the attenuated vaccine. Vaccination against influenza and COVID is recommended in pregnant women in any trimester or in the postpartum period within 6 months of birth if not vaccinated during pregnancy. Both vaccines can be given at the same time. (10) Measles, mumps and rubella vaccine (MMR).- Two doses of MMR vaccine. The first at age 12 months and the second at age 3–4 years. The quadrivalent MMRV vaccine may be administered for the second dose. In susceptible patients outside the specified ages, vaccination with 2 doses of MMR at least 1 month apart is recommended. (11) Varicella vaccine (Var).- Two doses: the first one at 15 months (although it is possible to administer from age 12 months) and the second at age 3–4 years. The quadrivalent vaccine (MMRV) may be used for the second dose. Susceptible patients outside the specified ages will be vaccinated with 2 doses of monovalent Var vaccine at least 1 month apart, with a recommended 12-week interval between doses in children aged less than 13 years. (12) SARS-CoV-2 vaccine.- One dose during pregnancy in any trimester. If pregnant women have been vaccinated before or had the infection, the vaccine should be given at least 3 months after the last exposure event. Vaccination in the postpartum period within 6 months of delivery is also indicated if not performed during the pregnancy. The vaccine can be given at the same time as the influenza or Tdap vaccines. (13) Human papillomavirus vaccine (HPV).- Universal routine vaccination against HPV in children of any sex at age 10–12 years with 2 doses. The vaccines currently available are HPV2 and HPV9. The higher valency vaccine (HPV9) is recommended. Vaccination schedules: 2-dose series (at 0 and 6 months) between 9 and 14 years and 3-dose series (at 0, 1–2 [depending on vaccine] and 6 months) in individuals aged 15 years or older. It can be administered at the same time as the MenC, MenACWY, hepatitis A and B and Tdap vaccines. There are no data for administration with the varicella vaccine, although it should not cause any problems. (14) Respiratory syncytial virus (RSV).- Pregnant women should receive the RSVPreF vaccine (Abrysvo) between 24 and 36 weeks of gestation, preferably between weeks 32 and 36. The vaccine is not available for the 2023−2024 season. Administration of 1 dose of nirsevimab (an anti-RSV antibody) is recommended in all neonates born during the RSV season (October-March) and infants aged less than 6 months (born between April and September) at the beginning of the season. Immunization schedule of the Spanish Association of Pediatrics: 2024 recommendations. Routine immunization. (1) Hepatitis B vaccine (HB).- Three doses of hexavalent vaccine at 2, 4 and 11 months. Unvaccinated children and adolescents should be given 3 doses of monovalent vaccine on a 0, 1 and 6-month schedule. (2) Diphtheria, tetanus and acellular pertussis vaccine (DTaP/Tdap).- Five doses: primary vaccination with 2 doses, at 2 and 4 months, and booster at 11 months (third dose) with DTaP-IPV-Hib-HB (hexavalent) vaccine; at 6 years (fourth dose) with the standard load vaccine (DTaP-IPV), preferable to the low diphtheria and pertussis antigen load vaccine (Tdap-IPV), and at 12–14 years (fifth dose) with Tdap. In children previously vaccinated with the 3 + 1 schedule (at 2, 4, 6 and 18 months), it is possible to use the Tdap for the booster at age 6 years, as they do not need additional doses of IPV. Administration of Tdap is recommended in each pregnancy between weeks 27 and 36 of gestation, preferably weeks 27−28. In the case of probable preterm labour, it can be administered from week 20, after performance of the high-resolution foetal ultrasound scan. (3) Inactivated poliovirus vaccine (IPV).- Four doses: primary vaccination with 2 doses, at 2 and 4 months, and booster doses at 11 months (with hexavalent vaccine) and 6 years (with DTaP-IPV or Tdap-IPV). Children previously vaccinated with the 3 + 1 schedule (at 2, 4, 6 and 18 months), require no additional doses of IPV. Children from countries that use the oral poliovirus vaccine (OPV) who have been vaccinated with 2 or 3 doses of the bivalent OPV vaccine exclusively (it was in April 2016 that the global switch from the bivalent OPV to the trivalent IPV started, as recommended by the WHO) should be given at least 2 doses of IPV at least 6 months apart to guarantee protection against poliovirus type 2. (4) Haemophilus influenzae type b conjugate vaccine (Hib).- Three doses: primary vaccination at 2 and 4 months and booster dose at 11 months with hexavalent vaccine. (5) Pneumococcal conjugate vaccine (PCV).- Three or four doses: 2 + 1 series with PCV15 (at 2, 4 and 11 months) or 3 + 1 series with PCV20 (at 2, 4, 6 and 11 months), when available. (6) Rotavirus vaccine (RV).- Two or three doses of rotavirus vaccine: at 2 and 3–4 months with the monovalent vaccine or at 2, 3 and 4 months or 2, 3–4 and 5–6 months with the pentavalent vaccine. To minimise the already low risk of intussusception, vaccination must start between 6 and 12 weeks of life and be completed by 24 weeks for the monovalent vaccine and 33 weeks for the pentavalent vaccine. Doses must be given at least 4 weeks apart. Both vaccines may be given at the same time as any other vaccine (with the exception of the oral poliovirus vaccine, which is not currently distributed in Spain). (7) Meningococcal B vaccine (MenB).- 4CMenB. Three doses: start at age 2 months, with a series of 2 doses 2 months apart and a booster starting from age 12 months and at least 6 months after the last dose in the primary series. Administration of the 4CMenB at the same time as other vaccines in the schedule is recommended. However, if either the clinician or the family prefer not to administer it at the same time, it can be given after any interval of time, although this has the drawback of delaying the protection provided by the vaccine. For all other age groups, the indication of vaccination with either vaccine (4CMenB or MenB-fHbp) is determined on a case-by-case basis, always adhering to the minimum age authorised for each vaccine. (8) Meningococcal ACWY conjugate vaccine (MenACWY).- One dose of conjugate MenACWY-TT at age 4 months and a booster dose at age 12 months. In adolescence (11–13 years), administration of 1 dose of MenACWY is recommended, in addition to catch-up vaccination through age 18 years. In autonomous communities where the MenACWY vaccine is not included in the routine immunization schedule at 4 and 12 months, if parents choose not to administer it, the MenC-TT vaccine funded by the regional government must be administered instead. For all other age groups, the decision to vaccinate must be made on a case-by-case basis. (9) Influenza vaccine.- recommended in all children aged 6–59 months with administration of an inactivated vaccine via the intramuscular route (some can be administered via deep subcutaneous injection) or, from age 2 years, preferably with the intranasal live attenuated vaccine. A single dose should be given from age 6 months, except in children aged less than 9 years in risk groups, who should be given 2 doses 4 weeks apart if it is the first time they are vaccinated against influenza. The dose is 0.5 mL delivered intramuscularly in the case of the inactivated vaccine and 0.1 mL in each nostril in the case of the attenuated vaccine. Vaccination against influenza and COVID is recommended in pregnant women in any trimester or in the postpartum period within 6 months of birth if not vaccinated during pregnancy. Both vaccines can be given at the same time. (10) Measles, mumps and rubella vaccine (MMR).- Two doses of MMR vaccine. The first at age 12 months and the second at age 3–4 years. The quadrivalent MMRV vaccine may be administered for the second dose. In susceptible patients outside the specified ages, vaccination with 2 doses of MMR at least 1 month apart is recommended. (11) Varicella vaccine (Var).- Two doses: the first one at 15 months (although it is possible to administer from age 12 months) and the second at age 3–4 years. The quadrivalent vaccine (MMRV) may be used for the second dose. Susceptible patients outside the specified ages will be vaccinated with 2 doses of monovalent Var vaccine at least 1 month apart, with a recommended 12-week interval between doses in children aged less than 13 years. (12) SARS-CoV-2 vaccine.- One dose during pregnancy in any trimester. If pregnant women have been vaccinated before or had the infection, the vaccine should be given at least 3 months after the last exposure event. Vaccination in the postpartum period within 6 months of delivery is also indicated if not performed during the pregnancy. The vaccine can be given at the same time as the influenza or Tdap vaccines. (13) Human papillomavirus vaccine (HPV).- Universal routine vaccination against HPV in children of any sex at age 10–12 years with 2 doses. The vaccines currently available are HPV2 and HPV9. The higher valency vaccine (HPV9) is recommended. Vaccination schedules: 2-dose series (at 0 and 6 months) between 9 and 14 years and 3-dose series (at 0, 1–2 [depending on vaccine] and 6 months) in individuals aged 15 years or older. It can be administered at the same time as the MenC, MenACWY, hepatitis A and B and Tdap vaccines. There are no data for administration with the varicella vaccine, although it should not cause any problems. (14) Respiratory syncytial virus (RSV).- Pregnant women should receive the RSVPreF vaccine (Abrysvo) between 24 and 36 weeks of gestation, preferably between weeks 32 and 36. The vaccine is not available for the 2023−2024 season. Administration of 1 dose of nirsevimab (an anti-RSV antibody) is recommended in all neonates born during the RSV season (October-March) and infants aged less than 6 months (born between April and September) at the beginning of the season.](https://static.elsevier.es/multimedia/23412879/0000010000000001/v1_202401141514/S2341287923002843/v1_202401141514/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w95erwEulN6Tmh1xJpRhO+VE=)
![Immunization schedule of the Spanish Association of Pediatrics: 2024 recommendations. Risk groups. (1) Hepatitis B vaccine (HB).- Children of HBsAg-positive mothers will be given 1 dose of vaccine and 1 dose of hepatitis B immune globulin (HBIG) (0.5 mL) within 12 h of birth. In the case of unknown maternal serologic status, children will receive the vaccine within 12 h of birth, followed by 0.5 mL of HBIG, preferably within 72 h of birth, if maternal HBsAg-positive status is confirmed. Infants vaccinated at birth will adhere to the routine schedule for the first year of life, and thus will receive 4 doses of HB vaccine. There are other risk groups. (2) Haemophilus influenzae type b vaccine (Hib).- Vaccination in children aged more than 59 months is unnecessary, except in those belonging to risk groups: anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, infection by HIV or history of invasive disease by H influenzae. In in unvaccinated or partially vaccinated children younger than 59 months, vaccinate according to the accelerated or catch-up vaccination schedule of the CAV-AEP. (3) Pneumococcal polysaccharide vaccine (PPSV).- The 23-valent vaccine (PPSV23) is indicated in children aged more than 2 years fully vaccinated with conjugate vaccine (PCV13 or PCV15) and with any disease increasing the risk of pneumococcal infection (1 or 2 doses, depending on risk factor); it should be given at least 8 weeks apart from the last dose of conjugate vaccine. When we have the 20-valent conjugate vaccine (PCV20), it will replace the dose of PPSV23 in children vaccinated with PCV13 or PCV15. In children who have been fully vaccinated with PCV20 (primary series and booster), administration of PPSV23 is not necessary. (4) Meningococcal B vaccine (MenB).- 4CMenB. Recommended in risk groups at any age from 1 year (infants under 1 year will be vaccinated according to the routine schedule): anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, haematopoietic stem cell transplant recipients, infection by HIV, prior episode of invasive meningococcal disease (IMD) caused by any serogroup and contacts of an index case of IMD caused by serogroup B in the context of an outbreak. Subsequently, with the exception of children aged less than 2 years or with a history of IMD, 1 dose of MenB should be given one year after completion of the primary series and every 5 years thereafter. In the context of an outbreak of IMD caused by group B, patients in risk groups should be given a booster dose if at least 1 year has elapsed from completion of the primary vaccination series. (5) Meningococcal ACWY conjugate vaccine(MenACWY).- The MenACWY continues to be particularly recommended for children and adolescents who are going to move to countries where this vaccine is indicated at the corresponding age (Canada, USA, Argentina, Chile, Saudi Arabia, Australia, Andorra, Austria, Belgium, Cyprus, Slovakia, Greece, Ireland, Italy, Malta, Netherlands, United Kingdom, Czech Republic, San Marino, Switzerland) and children in risk groups: anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, haematopoietic stem cell transplant recipients, infection by HIV, prior episode of invasive meningococcal disease (IMD) caused by any serogroup and contacts of an index case of IMD caused by serogroup A, C, W or Y in the context of an outbreak. Primary vaccination at any age with 2 doses at least 2 months apart. If the risk persists, administration of a booster dose is recommended every 3 years in children aged less than 7 years and every 5 years in older children. Travellers to Mecca or the African meningitis belt in the dry season must also be vaccinated with MenACWY. (6) Influenza vaccine.- Recommended for all risk groups and household contacts from age 6 months. The risk groups relevant to this vaccine can be found in the document outlining the recommendations of the CAV-AEP for the 2023−2024 season. (7) SARS-CoV-2 vaccine.- According to the recommendations of the Public Health Commission of Spain concerning vaccination against COVID-19 for the 2023−2024 season, vaccination is indicated from age 6 months in individuals with diseases considered a high or very high risk, receiving immunosuppressive treatment or who are household contacts of at-risk individuals, as well as individuals aged 5 years or older living in residential facilities or institutionalised for prolonged periods. Monovalent vaccines against the omicron XBB.1.5 variants should be used: Comirnaty XBB.1.5 (preparations containing 3 µg [age 6 months–4 years], 10 µg [age 5−11 years] or 30 µg [age ≥ 12 years]) or Spikevax XBB.1.5 (available as 0.1 mg/mL multidose vial to deliver 10 doses of 2.5 mL/25 µg [age 6 months–11 years] or 5 doses of 0.5 mL/50 µg [age ≥ 11 years]). Primary vaccination in individuals aged more than 6 months who have had the infection: single dose, at least 3 months after the infection, except in severely immunosuppressed patients, who should receive a second dose at least 3 months after the first one. Primary vaccination in individuals with no history of infection: for those aged 5 years or older, a single dose; for children aged 6 months to 4 years, 3 doses at 0, 3 and 8 weeks of Comirnaty XBB.1.5 3 µg or 2 doses of Spikevax XBB.1.5 (0.25 mL/25 µg) at 0 and 28 days. In children aged 6 months to 4 years who are partially vaccinated, complete the series with one of the new monovalent vaccines. Annual seasonal dose (autumn-winter 2023−2024) in risk groups: single dose, independently of the number of doses received in the past, in those previously vaccinated or with a previous history of SARS-CoV-2 infection at least 3 months after the last dose of vaccine or episode of infection. The risk groups can be consulted in the recommendations published by the Ministry of Health and in the online manual of immunizations of the CAV-AEP. (8) Human papillomavirus vaccine (VPH).- Vaccination is indicated from age 9 years, always with 3 doses, in immunosuppressed individuals. Consult the Manual of immunizations for other risk groups. (9). Hepatitis A vaccine (HA).- The pre-exposure and post-exposure risk groups are detailed in our Manual. Infants aged 6–11 months traveling to risk areas can be given the vaccine, but it will not count as a valid dose toward the routine vaccination series, which will have to start over from age 12 months. (10)Respiratory syncytial virus antibody(RSVAb).- Administration of nirsevimab (anti-VRS antibody) is recommended annually (for 2 seasons) in children aged less than 2 years with underlying disease increasing the risk of severe RSV infection, preferably just before the usual start of the RSV season (October). Immunization schedule of the Spanish Association of Pediatrics: 2024 recommendations. Risk groups. (1) Hepatitis B vaccine (HB).- Children of HBsAg-positive mothers will be given 1 dose of vaccine and 1 dose of hepatitis B immune globulin (HBIG) (0.5 mL) within 12 h of birth. In the case of unknown maternal serologic status, children will receive the vaccine within 12 h of birth, followed by 0.5 mL of HBIG, preferably within 72 h of birth, if maternal HBsAg-positive status is confirmed. Infants vaccinated at birth will adhere to the routine schedule for the first year of life, and thus will receive 4 doses of HB vaccine. There are other risk groups. (2) Haemophilus influenzae type b vaccine (Hib).- Vaccination in children aged more than 59 months is unnecessary, except in those belonging to risk groups: anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, infection by HIV or history of invasive disease by H influenzae. In in unvaccinated or partially vaccinated children younger than 59 months, vaccinate according to the accelerated or catch-up vaccination schedule of the CAV-AEP. (3) Pneumococcal polysaccharide vaccine (PPSV).- The 23-valent vaccine (PPSV23) is indicated in children aged more than 2 years fully vaccinated with conjugate vaccine (PCV13 or PCV15) and with any disease increasing the risk of pneumococcal infection (1 or 2 doses, depending on risk factor); it should be given at least 8 weeks apart from the last dose of conjugate vaccine. When we have the 20-valent conjugate vaccine (PCV20), it will replace the dose of PPSV23 in children vaccinated with PCV13 or PCV15. In children who have been fully vaccinated with PCV20 (primary series and booster), administration of PPSV23 is not necessary. (4) Meningococcal B vaccine (MenB).- 4CMenB. Recommended in risk groups at any age from 1 year (infants under 1 year will be vaccinated according to the routine schedule): anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, haematopoietic stem cell transplant recipients, infection by HIV, prior episode of invasive meningococcal disease (IMD) caused by any serogroup and contacts of an index case of IMD caused by serogroup B in the context of an outbreak. Subsequently, with the exception of children aged less than 2 years or with a history of IMD, 1 dose of MenB should be given one year after completion of the primary series and every 5 years thereafter. In the context of an outbreak of IMD caused by group B, patients in risk groups should be given a booster dose if at least 1 year has elapsed from completion of the primary vaccination series. (5) Meningococcal ACWY conjugate vaccine(MenACWY).- The MenACWY continues to be particularly recommended for children and adolescents who are going to move to countries where this vaccine is indicated at the corresponding age (Canada, USA, Argentina, Chile, Saudi Arabia, Australia, Andorra, Austria, Belgium, Cyprus, Slovakia, Greece, Ireland, Italy, Malta, Netherlands, United Kingdom, Czech Republic, San Marino, Switzerland) and children in risk groups: anatomic or functional asplenia, complement deficiency, treatment with eculizumab or ravulizumab, haematopoietic stem cell transplant recipients, infection by HIV, prior episode of invasive meningococcal disease (IMD) caused by any serogroup and contacts of an index case of IMD caused by serogroup A, C, W or Y in the context of an outbreak. Primary vaccination at any age with 2 doses at least 2 months apart. If the risk persists, administration of a booster dose is recommended every 3 years in children aged less than 7 years and every 5 years in older children. Travellers to Mecca or the African meningitis belt in the dry season must also be vaccinated with MenACWY. (6) Influenza vaccine.- Recommended for all risk groups and household contacts from age 6 months. The risk groups relevant to this vaccine can be found in the document outlining the recommendations of the CAV-AEP for the 2023−2024 season. (7) SARS-CoV-2 vaccine.- According to the recommendations of the Public Health Commission of Spain concerning vaccination against COVID-19 for the 2023−2024 season, vaccination is indicated from age 6 months in individuals with diseases considered a high or very high risk, receiving immunosuppressive treatment or who are household contacts of at-risk individuals, as well as individuals aged 5 years or older living in residential facilities or institutionalised for prolonged periods. Monovalent vaccines against the omicron XBB.1.5 variants should be used: Comirnaty XBB.1.5 (preparations containing 3 µg [age 6 months–4 years], 10 µg [age 5−11 years] or 30 µg [age ≥ 12 years]) or Spikevax XBB.1.5 (available as 0.1 mg/mL multidose vial to deliver 10 doses of 2.5 mL/25 µg [age 6 months–11 years] or 5 doses of 0.5 mL/50 µg [age ≥ 11 years]). Primary vaccination in individuals aged more than 6 months who have had the infection: single dose, at least 3 months after the infection, except in severely immunosuppressed patients, who should receive a second dose at least 3 months after the first one. Primary vaccination in individuals with no history of infection: for those aged 5 years or older, a single dose; for children aged 6 months to 4 years, 3 doses at 0, 3 and 8 weeks of Comirnaty XBB.1.5 3 µg or 2 doses of Spikevax XBB.1.5 (0.25 mL/25 µg) at 0 and 28 days. In children aged 6 months to 4 years who are partially vaccinated, complete the series with one of the new monovalent vaccines. Annual seasonal dose (autumn-winter 2023−2024) in risk groups: single dose, independently of the number of doses received in the past, in those previously vaccinated or with a previous history of SARS-CoV-2 infection at least 3 months after the last dose of vaccine or episode of infection. The risk groups can be consulted in the recommendations published by the Ministry of Health and in the online manual of immunizations of the CAV-AEP. (8) Human papillomavirus vaccine (VPH).- Vaccination is indicated from age 9 years, always with 3 doses, in immunosuppressed individuals. Consult the Manual of immunizations for other risk groups. (9). Hepatitis A vaccine (HA).- The pre-exposure and post-exposure risk groups are detailed in our Manual. Infants aged 6–11 months traveling to risk areas can be given the vaccine, but it will not count as a valid dose toward the routine vaccination series, which will have to start over from age 12 months. (10)Respiratory syncytial virus antibody(RSVAb).- Administration of nirsevimab (anti-VRS antibody) is recommended annually (for 2 seasons) in children aged less than 2 years with underlying disease increasing the risk of severe RSV infection, preferably just before the usual start of the RSV season (October).](https://static.elsevier.es/multimedia/23412879/0000010000000001/v1_202401141514/S2341287923002843/v1_202401141514/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w95erwEulN6Tmh1xJpRhO+VE=)


