Rare diseases are a challenge for public health due to the lack of information on their magnitude. These include inborn errors of metabolism. The objective of this study was to assess the quality of life and social, health, economic, and educational needs of a group of paediatric patients with inborn errors of metabolism attended to in a hospital.
Material and methodA questionnaire was developed based on the needs and expectations, based mainly on the Andalusian Plan for Rare Diseases. An analysis was performed on the variables of health, socioeconomic, and educational needs of 65 paediatric patients with inborn errors of metabolism.
ResultsThe respondents showed few possibilities to cope with medication (61%), special diet (86%), and other health benefits (79%). Just under half of them (43%) believed that the quality of family life had been greatly reduced since the onset of the disease. The main caregiver was the mother in 61.5% of cases, compared to 1.5% of cases in which it was the father. The primary caregivers had to reduce their working hours or give up their job in 77% of cases.
ConclusionsThe multidisciplinary treatment is affected by the inability of families to cope with a high cost, as well as with difficult access to these resources. In addition, there is great impact on the quality of life of patients, and their caregivers. Therefore, there is a need to evaluate the results of government health and socio-economic support plans for patients with rare diseases, and make a real response to their needs.
Las enfermedades raras suponen un reto para la salud pública debido a la escasa información sobre su magnitud. Entre ellas destacan los errores congénitos del metabolismo. El objetivo de este estudio fue valorar la calidad de vida y las posibles necesidades de un grupo de pacientes pediátricos con errores congénitos del metabolismo asistidos en un hospital de tercer nivel, y de sus familiares o cuidadores principales.
Material y métodoSe desarrolló un cuestionario basado en las necesidades y expectativas recogidas fundamentalmente en el Plan Andaluz para las Enfermedades Raras. Se analizaron variables de los órdenes sociosanitario, económico y educativo en 65 pacientes pediátricos con errores congénitos del metabolismo.
ResultadosLos encuestados manifestaron escasas posibilidades para afrontar el gasto de la medicación (61%), alimentación especial (86%) y otras prestaciones sanitarias (79%). El 43% consideraron que la calidad de vida familiar se afectó bastante desde la aparición de la enfermedad. En el 61,5% la cuidadora principal fue la madre, frente al 1,5% de casos en los que fue el padre. El cuidador principal redujo su jornada laboral o abandonó su trabajo en el 77% de los casos.
ConclusionesEl tratamiento multidisciplinar se ve afectado por la imposibilidad de las familias para hacer frente a su elevado coste, junto a una difícil accesibilidad a dichos recursos. Además, existe gran repercusión en la calidad de vida de los pacientes y sus cuidadores. Por tanto, deberían evaluarse los resultados de los planes gubernamentales de apoyo sanitario y socioeconómico a pacientes con enfermedades raras, y conseguir una respuesta real a sus necesidades.
The term rare diseases (RDs) refers to a large and heterogeneous group of diseases of which we have little knowledge due to their complex course, as they develop slowly with symptoms that are often nonspecific and highly variable, and their very low prevalence (<5/10000 individuals). Most have a genetic aetiology and are chronic and degenerative, so that they are disabling or limiting to a variable degree. The need for early diagnosis and multidisciplinary care in these diseases is increasingly evident.1,2 All of these challenges are compounded by other factors that hinder their management, such as the low availability and affordability of specific drugs and foods required for their treatment, the scarce knowledge of health professionals about RDs, and the lack of specific health care protocols.3,4
Given the health care, social and educational impact of these diseases, we need to assess whether nationwide government interventions succeed in improving the life of affected individuals and their families and meet their basic and special needs.5 Generally speaking, the social and economic impact of a RD on patients and their families have been addressed by patient organisations.6 Among the most salient studies on the subject conducted in Spain are the Studies on the situation of the Social and Health Care Needs of Individuals with Rare Diseases in Spain (Estudio sobre la situación de las Necesidades Sociosanitarias de las personas con Enfermedades Raras en España, ENSERio) 1 and 2, funded by the Federación Española de Enfermedades Raras (Spanish Federation on Rare Diseases) that assessed the economic, social, educational, health care, psychological and employment dimensions.3
The implementation of newborn screening programmes has allowed the early diagnosis and treatment of a few RDs. However, there are multiple factors that impair quality of life in these patients, which has led some authors to investigate their health-related7–9 and overall10,11 quality of life. There are also important psychological and psychiatric factors in some of these diseases,12,13 not forgetting the impact of psychosocial factors in the families of affected individuals.14 Several initiatives have been developed to explore and address needs in these areas that have so far been neglected in RDs, such the Rare Disease Strategy of the National Health System of the Spanish Ministry of Health (Estrategia en Enfermedades Raras del Sistema Nacional de Salud, 2013), and the Plan for the Care of Individuals Affected by Rare Diseases (Plan de Atención a personas afectadas por Enfermedades Raras, PAER) of the Department of Health of Andalusia,2 whose general purpose is to ensure adequate planning and management of health care resources used for the care of individuals with RDs and their families to guarantee a high quality of care and equal access.
The aim of our study was to assess paediatric patients with metabolic RDs followed up at one tertiary care hospital and their families or main caregivers.
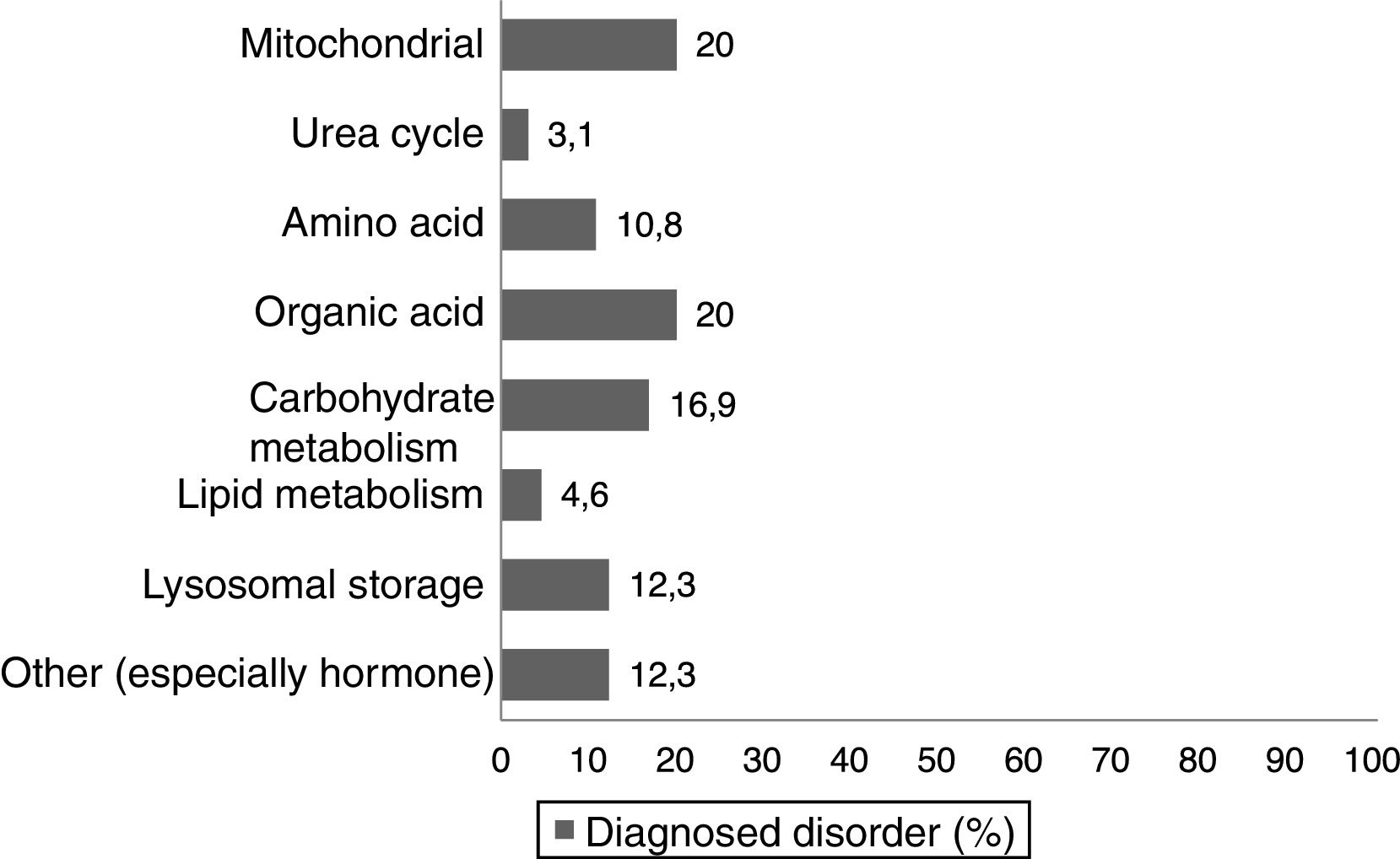
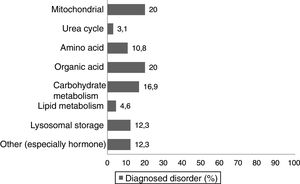
Materials and methodsWe conducted a nonexperimental, cross-sectional descriptive study. We asked a total of 74 parents and/or legal guardians of paediatric patients with different inborn errors of metabolism (Fig. 1) managed in the paediatric clinic of a tertiary care hospital to be interviewed face-to-face or on the telephone. The final sample included 65 respondents, as 5 individuals declined to participate in the survey and we were unable to reach another 4 for the survey. The questionnaire was completed by the parent/legal guardian, or by the patient if the patient was free of intellectual or physical disability. The study was approved by the competent Ethics Committee.
We developed a questionnaire consisting of 70 closed-ended, single-answer items based on the needs expressed during patient visits and related to the variables gathered in the PAER 2008–2012 plan2 regarding needs and expectations in the health care, psychosocial, educational and economic (funding of diet and medication) dimensions. We collected data on demographic and other variables in the following areas:
Health care dimension: newborn screening, treatments used, special medication and diet, other health care services, financial cost and accessibility of these resources. We also analysed variables related to disability, dependence, financial assistance, health care, lack of coverage, scientific knowledge of the disease, help from organisations and research.
Educational dimension: type of education, school-based and home-based support, health professionals involved in school, bullying.
Psychosocial dimension: quality of life of paediatric patients and their caregivers, main caregiver categories, psychological support, family structure, employment, and other variables related to the need to care for the affected individual.
Statistical analysisWe performed a descriptive analysis of all the variables under study, expressing quantitative variables as mean, standard deviation, median and interquartile range, and qualitative variables as absolute and relative frequencies. We also compared the proportions of some variables of interest by means of the chi square test (χ2) for contingency tables. In the case of 2×2 contingency tables, we used the χ2 with the Yates correction or, if any of the expected frequencies was less than or equal to 5, the Fisher exact test. We performed the analysis with the software SPSS 18.0.
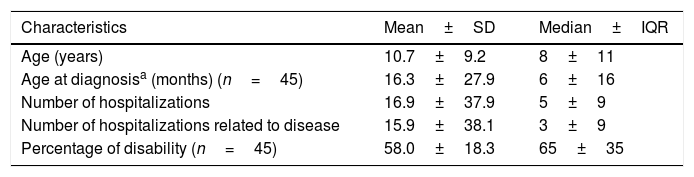
ResultsHealth careIn our study, a significant percentage of paediatric patients (78.5%) had diseases that could not be diagnosed through neonatal screening, as they were not included in the early-detection Newborn Endocrine-Metabolic Disease Screening Programme of Andalusia. This screening usually includes diseases in which early intervention allows modification of the course of disease, reducing morbidity and mortality and the incidence of potential disabilities associated with the disease. Of the patients in whom screening prompted diagnosis, 7.7% experienced the onset before the results of screening became available. Most of these patients received a genetic diagnosis, and prenatal diagnosis of the same disease was made in 12% of their families in subsequent pregnancies (Table 1).
of the paediatric patients with inborn errors of metabolism included in the sample (N=65).
| Characteristics | Mean±SD | Median±IQR |
|---|---|---|
| Age (years) | 10.7±9.2 | 8±11 |
| Age at diagnosisa (months) (n=45) | 16.3±27.9 | 6±16 |
| Number of hospitalizations | 16.9±37.9 | 5±9 |
| Number of hospitalizations related to disease | 15.9±38.1 | 3±9 |
| Percentage of disability (n=45) | 58.0±18.3 | 65±35 |
| Diagnosis | Count | Percentage | |
|---|---|---|---|
| Newborn screening | 14 | 21.5 | |
| Clinical manifestations (n=51) | In childa | 46 | 90.2 |
| In relative | 5 | 9.8 | |
| Genetic diagnosis | 60 | 92.3 | |
| Prenatal diagnosis (n=59) | 7 | 11.9 |
IQR, interquartile range; SD, standard deviation.
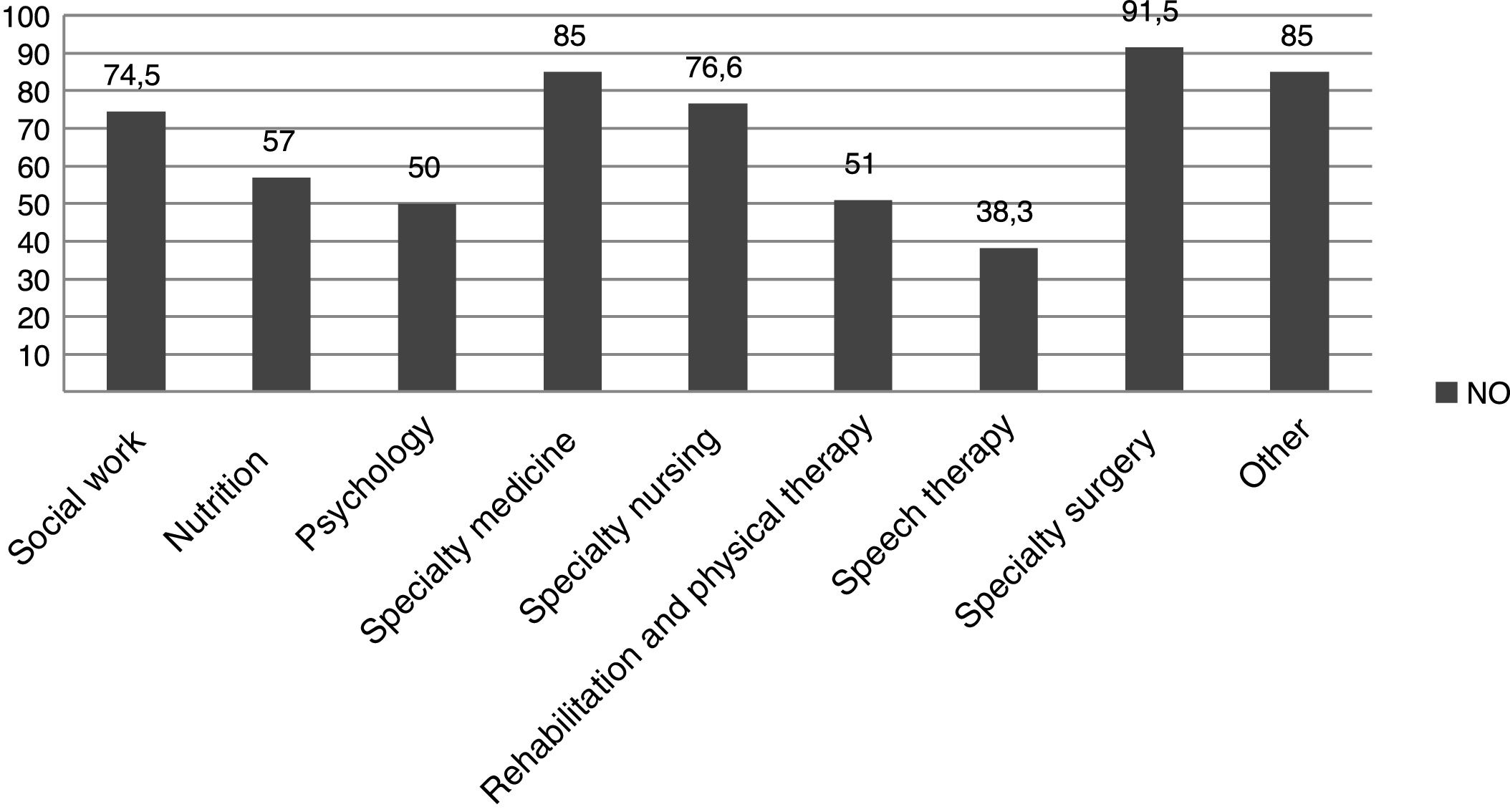
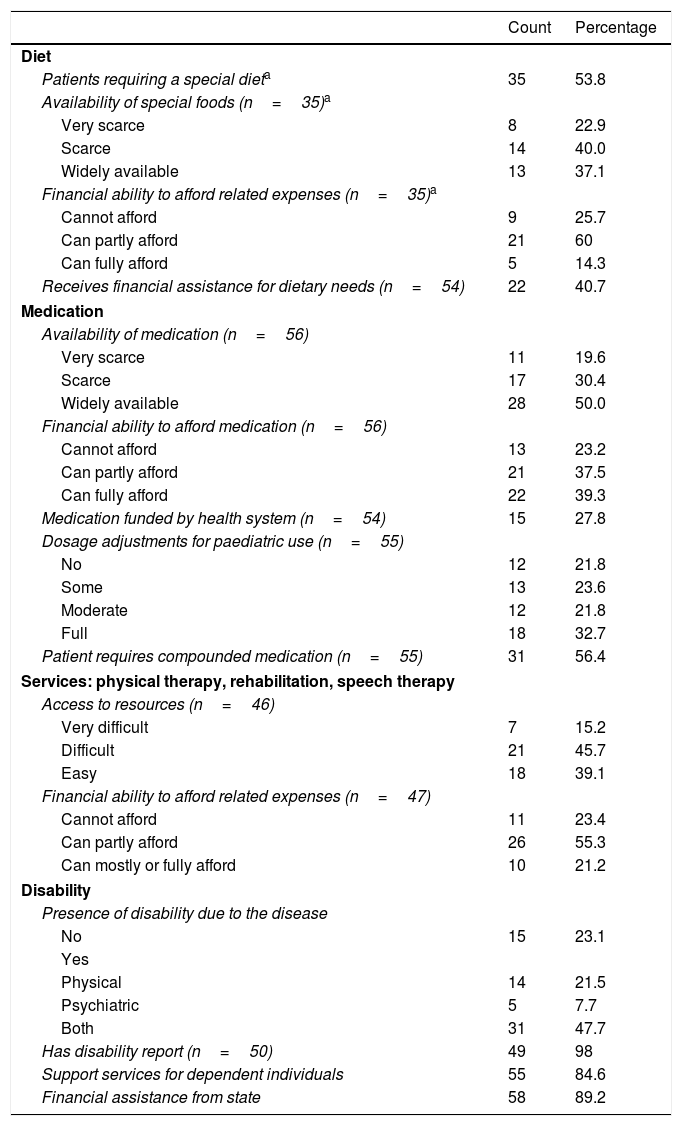
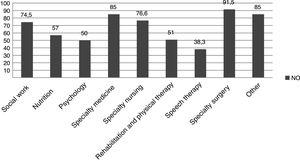
When it came to treatment, families reported having difficulties regarding the low availability and accessibility of dietary products (needed by 54% of the sample), medication and other resources (Table 2). We found a high percentage of patients that required drugs and products that were not funded by the health care system, and a frequent need to resort to compounding for the appropriate medication of the patients (Table 2), in addition to other services not covered by public health (Fig. 2). These patients had some degree of physical and/or psychiatric impairment, which was assessed objectively by external professionals that produced a report on the disability rate of patients (Table 1), based on which most of them received some type of financial assistance from the state (Table 2).
Analysis of health care variables: accessibility of resources for the surveyed families of paediatric patients with inborn errors of metabolism (N=65).
| Count | Percentage | |
|---|---|---|
| Diet | ||
| Patients requiring a special dieta | 35 | 53.8 |
| Availability of special foods (n=35)a | ||
| Very scarce | 8 | 22.9 |
| Scarce | 14 | 40.0 |
| Widely available | 13 | 37.1 |
| Financial ability to afford related expenses (n=35)a | ||
| Cannot afford | 9 | 25.7 |
| Can partly afford | 21 | 60 |
| Can fully afford | 5 | 14.3 |
| Receives financial assistance for dietary needs (n=54) | 22 | 40.7 |
| Medication | ||
| Availability of medication (n=56) | ||
| Very scarce | 11 | 19.6 |
| Scarce | 17 | 30.4 |
| Widely available | 28 | 50.0 |
| Financial ability to afford medication (n=56) | ||
| Cannot afford | 13 | 23.2 |
| Can partly afford | 21 | 37.5 |
| Can fully afford | 22 | 39.3 |
| Medication funded by health system (n=54) | 15 | 27.8 |
| Dosage adjustments for paediatric use (n=55) | ||
| No | 12 | 21.8 |
| Some | 13 | 23.6 |
| Moderate | 12 | 21.8 |
| Full | 18 | 32.7 |
| Patient requires compounded medication (n=55) | 31 | 56.4 |
| Services: physical therapy, rehabilitation, speech therapy | ||
| Access to resources (n=46) | ||
| Very difficult | 7 | 15.2 |
| Difficult | 21 | 45.7 |
| Easy | 18 | 39.1 |
| Financial ability to afford related expenses (n=47) | ||
| Cannot afford | 11 | 23.4 |
| Can partly afford | 26 | 55.3 |
| Can mostly or fully afford | 10 | 21.2 |
| Disability | ||
| Presence of disability due to the disease | ||
| No | 15 | 23.1 |
| Yes | ||
| Physical | 14 | 21.5 |
| Psychiatric | 5 | 7.7 |
| Both | 31 | 47.7 |
| Has disability report (n=50) | 49 | 98 |
| Support services for dependent individuals | 55 | 84.6 |
| Financial assistance from state | 58 | 89.2 |
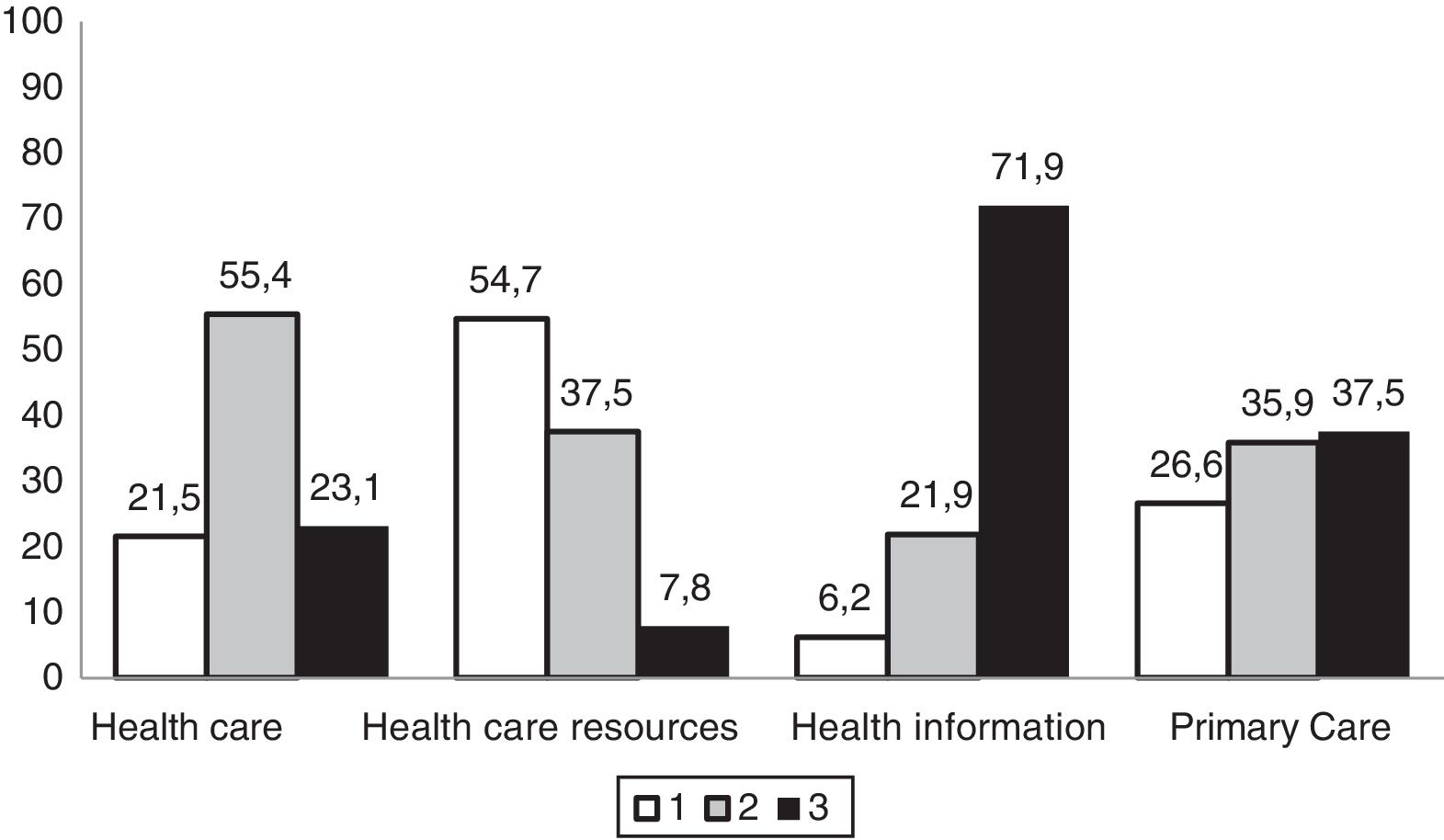
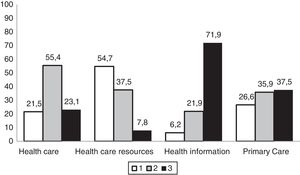
In general, respondents perceived the health care received as good or very good. More than half believed that they had been receiving sufficient and understandable information about the disease, although the health care system had few resources for the management of RDs. They rated the competence of primary care health care providers in managing the disease in the patient as low (Fig. 3), and believed that they had little scientific knowledge of the disease (69.2%). On the other hand, up to 71% reported receiving sufficient and adequate support from specialty health care providers. Up to 54% of families were members of patient organisations related to the disease of their children, and most participated in research in RDs (69%). Twenty percent of patients had participated in a clinical trial focused on their disease.
Distribution of the rating of primary care services given by surveyed family members of paediatric patients with inborn errors of metabolism (N=65).
Health care: (1) poor/sufficient; (2) good/very good; (3) excellent.
Health care resources: (1) minimal/few; (2) sufficient; (3) plentiful.
Health information: (1) incomprehensible; (2) scarce; (3) understandable and adequate.
Primary care: (1) deficient; (2) barely adequate; (3) highly competent.
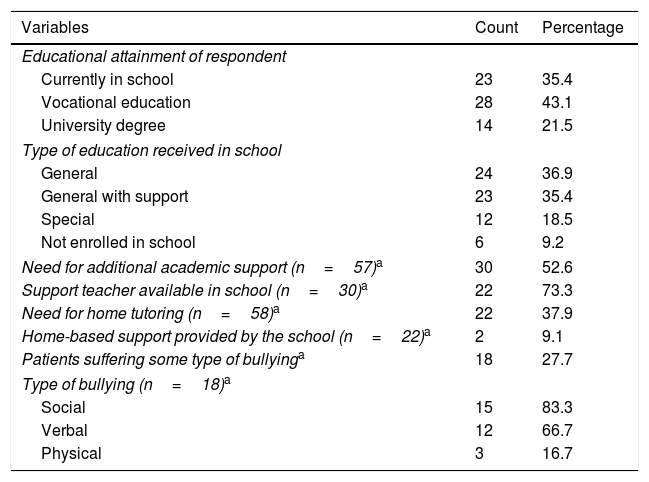
Most respondents had a vocational education, and 21.5% a university degree. We did not find statistically significant differences in the perception of health care based on parental educational attainment (χ2=1.667; P=.221). A high proportion of patients were enrolled in general education classrooms or in general education with additional support (72.3%): 52.6% of the patients required additional educational support, and more than half received this support from a support teacher on staff in their respective schools. In addition to the education received at school, 38% received tutoring at home (Table 3).
Assessment of socioeducational variables related to the services received from the schools attended by paediatric patients with inborn errors of metabolism (N=65).
| Variables | Count | Percentage |
|---|---|---|
| Educational attainment of respondent | ||
| Currently in school | 23 | 35.4 |
| Vocational education | 28 | 43.1 |
| University degree | 14 | 21.5 |
| Type of education received in school | ||
| General | 24 | 36.9 |
| General with support | 23 | 35.4 |
| Special | 12 | 18.5 |
| Not enrolled in school | 6 | 9.2 |
| Need for additional academic support (n=57)a | 30 | 52.6 |
| Support teacher available in school (n=30)a | 22 | 73.3 |
| Need for home tutoring (n=58)a | 22 | 37.9 |
| Home-based support provided by the school (n=22)a | 2 | 9.1 |
| Patients suffering some type of bullyinga | 18 | 27.7 |
| Type of bullying (n=18)a | ||
| Social | 15 | 83.3 |
| Verbal | 12 | 66.7 |
| Physical | 3 | 16.7 |
Most of the schools where the patients were enrolled did not have health professionals on staff that could tend to the specific needs of their disease (91%). Some schools had facilities that were not easily accessible for disabled individuals (23%). In general, the services received in the school were perceived as good, very good or excellent (66%), while 12% considered them very poor. Some patients with inborn errors of metabolism (IEMs) suffered bullying of some kind: social, verbal or physical (Table 3).
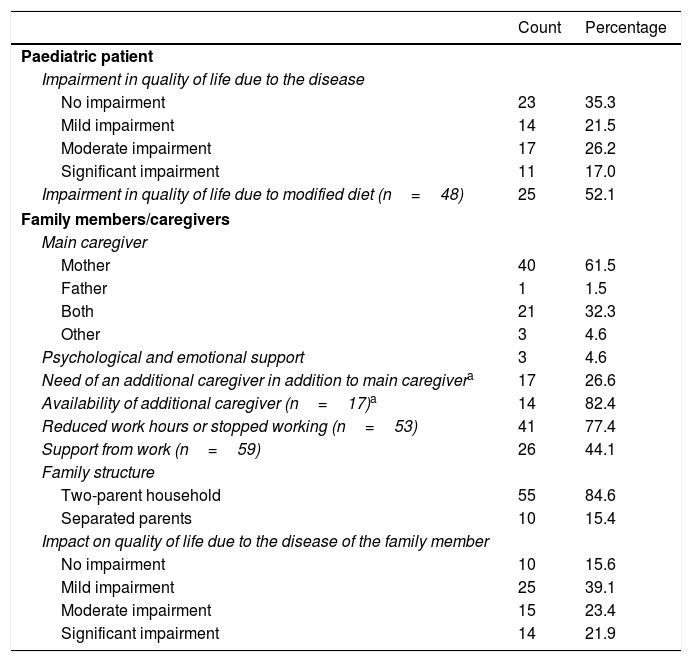
Quality of lifeThe quality of life of the patients declined significantly from the onset of symptoms in 43% of the sample, and even more in patients that required significant adjustments to their diet (52.1%) (Table 4). However, we did not find statistically significant differences in patient quality of life based on the need for a modified diet (χ2=0.083; P=.974).
Perceived quality of life of patients with inborn errors of metabolism in the study and their parents or caregivers (N=65).
| Count | Percentage | |
|---|---|---|
| Paediatric patient | ||
| Impairment in quality of life due to the disease | ||
| No impairment | 23 | 35.3 |
| Mild impairment | 14 | 21.5 |
| Moderate impairment | 17 | 26.2 |
| Significant impairment | 11 | 17.0 |
| Impairment in quality of life due to modified diet (n=48) | 25 | 52.1 |
| Family members/caregivers | ||
| Main caregiver | ||
| Mother | 40 | 61.5 |
| Father | 1 | 1.5 |
| Both | 21 | 32.3 |
| Other | 3 | 4.6 |
| Psychological and emotional support | 3 | 4.6 |
| Need of an additional caregiver in addition to main caregivera | 17 | 26.6 |
| Availability of additional caregiver (n=17)a | 14 | 82.4 |
| Reduced work hours or stopped working (n=53) | 41 | 77.4 |
| Support from work (n=59) | 26 | 44.1 |
| Family structure | ||
| Two-parent household | 55 | 84.6 |
| Separated parents | 10 | 15.4 |
| Impact on quality of life due to the disease of the family member | ||
| No impairment | 10 | 15.6 |
| Mild impairment | 25 | 39.1 |
| Moderate impairment | 15 | 23.4 |
| Significant impairment | 14 | 21.9 |
Some respondents believed the patient needed an additional caregiver (26.6%), as in most cases the daily care was provided by a family member. Many main caregivers, who were usually the mother (61.5%), had reduced their working hours or quit working altogether (77.4% of caregivers), and had not received support from their workplaces to balance work with caring for the patient (56%). The proportion that received psychological support from the health care system was 4.6%, and we did not find a significant association between this factor and perceived quality of life (χ2=0.229; P=1.000). The most frequent family structure was the two-parent household, and 15.4% of parents were separated (Table 4), of who 50% reported believing that the disease of their children contributed to the separation.
DiscussionOur study revealed health care, socioeducational and economic needs of paediatric patients with inborn errors of metabolism and their caregivers that were not considered in the PAER. Similarly, the findings of studies focused on health care and social factors, such as the ENSERio studies 1 and 2, have demonstrated the economic, social, educational, health care, psychological and occupational impact of these diseases in affected children and their families.
Unlike other studies focused on specific diseases, such as phenylketonuria,15 galactosaemia16 or mucopolysaccharidosis,17 our study included patients with a variety of metabolic diseases. We ought to underscore that we assessed the quality of life of patients and caregivers in relation to their specific diseases by means of the questionnaire used in our study, similar to other instruments: the Paediatric Quality of Life Inventory,11,18 the WHOQOL Questionnaire-10011 or the Child Health Questionnaire 28-item Parent Form.18 However, the fact that we investigated different forms of metabolic disease may have also been a limitation in our analysis, as the fulfilment of associated needs may vary widely between diseases. In our study, we sought to assess these aspects in all patients followed in a specialised metabolic disease unit, regardless of their specific diseases, and, as was the case of the PAER, whether those needs were met appropriately.
Five family members of the total possible participants declined to do the survey, and the reason given for refusal was the death of the patient. This very small number reduces the risk of nonresponse bias by which the survey data would show a greater-than-actual satisfaction.
On the other hand, in agreement with previous studies, our data revealed the limited knowledge of health professionals regarding these diseases, which is often associated with diagnostic delays.19 Even though prenatal testing in subsequent pregnancies could have a significant impact on family planning in families that have already had one child with an inborn error of metabolism, few of these families receive specialised genetic counselling.20 A similar situation has been reported in other highly developed countries.3,4,21,22 The ENSERio and ERES studies in Spain revealed that the time elapsed from the onset of symptoms to diagnosis of RDs is almost 5 years in the adult population and 1 year in the paediatric population, figures that, compared to the delays in diagnosis in other, common diseases (severe or not) is quite substantial. Specific training of health professionals in RDs could contribute to reducing diagnostic delays and their negative repercussions, such as delayed or inadequate treatment and performance of unnecessary tests.23,24
When it came to medication and other health care products, patients reported having little to no access to what they required. As reported in the previous literature, the most frequent difficulties were the high price of these products, their low availability, or even the impossibility of obtaining them in the country of residence of the patient.25 Respondents also reported that Social Security funded required medication and health products only in part, which was consistent with the findings of the study conducted by the Federación Española de Enfermedades Raras,3 although level of coverage of specific expenses was not specified. In the paediatric patients in our sample, the high proportion that required medication or special dietary products not covered by the health system was compounded by the amount of services that were not offered on a long-term basis in the Spanish public health system, such as nutritionists, psychologists to support the family after the diagnosis, rehabilitation, physical therapy or speech therapy (Table 2).
Eighty-nine percent of patients in our study received some type of financial assistance from the state, although in other surveys families have reported that this assistance is quite insufficient to meet the needs of the patients, especially when it comes to medication.4
In our sample, 21.5% of the families perceived the health care they received as poor or just sufficient, and 62.5% considered primary care services deficient or barely adequate (Fig. 3), which was consistent with other studies, such as the EurodisCare-3, which ranked Spain as the European country with the lowest satisfaction (20%) with health care services related to the management of RDs.21 In the ENSERio study, the percentage of dissatisfied participants even exceeded 47%, which could be explained by the more than 200 different diagnoses it contemplated, of which some have extremely low prevalences,3 compared to the sample of patients with 16 different RDs in the EurodisCare-3 study. This perception seems to be associated with the scarcity of resources available in the health care system to manage these diseases and the lack of reference centres in the geographical area of the patients. To this we must add the lack of specific protocols or management strategies, not forgetting the very limited or incomprehensible information that health professionals give these patients.26
The educational attainment of parents may have a significant impact on the treatment of their children, as it can lead to a lesser adherence to dietary measures or other treatments.27,28 In our study, caregivers with a university degree were the minority, and 35.4% of caregivers had an education at the primary school level. Most patients were in general education classrooms, with or without additional support from the school that they attended, and these patients were subject to some type of bullying. As a result, some authors consider that these children should attend schools offering special education programmes to reduce the social pressure they experience.27
Other authors have focused on aspects related to educational services that have an impact on the quality of life of patients with inborn errors of metabolism, such as attention problems, school problems, lower achievement motivation, lower social skills, lower sense of autonomy and lower self-esteem. Self-esteem and family cohesion are the areas with the highest impact on quality of life, sometimes exceeding the impact of physical impairment or pain.29 In adulthood, these patients are also likely to suffer from depressive moods, generalised anxiety, phobias, decreased positive affect, social immaturity and social withdrawal12,13 independently of the management of the disease.30 In our study, we did not analyse these educational aspects with that level of detail, but assessed the need of additional educational support, which was perceived as needed in more than half of the cases and was delivered at the home.
Nearly half of respondents considered that their quality of life had substantially declined from the onset of symptoms. More than 50% of patients that required a modified diet associated this decrease to their dietary needs, although we did not find a statistically significant association between the impairment in quality of life and the need of a special diet, probably because our sample was too small to detect it. In a study with a similar sample size, Bilginsoy et al.31 noted that the management of the diet and its impact on the parents’ social life could be considered among the chief sources of stress in the family. This study also suggested that the special dietary needs of children were a factor that contributed substantially to the decrease in quality of life in their caregivers (48.5%). This evinces the need to develop strategies to facilitate the integration of specialised dietary treatment in the routine of the household, promoting adequate adherence and improving the quality of life of the patient and the remaining household members.32
Of the patients in our sample, 56.8% considered that the onset of disease had had little impact on their quality of life. This could be because when patients start treatment early with medication and/or a specific diet, and depending on the type of RD and the acceptance of illness, the improved outcomes influence this perception.11
Of the surveyed family members, 45% considered that their quality of life had diminished significantly since the onset of disease in their children, which in many cases required an additional caregiver, a reduction of working hours or even quitting work to adapt their lives to care for the patient (Table 4). Dependency in these diseases involves the need for personal care assistance, which usually falls to the main caregivers with considerable repercussions in their social life, work and education, especially in women.3,6 In fact, some parents try multiple adaptive strategies to integrate the complex protocols for the management of their children's disease in the daily routine of the household.33
Some authors propose that parental quality of life may be the best indicator to assess how parents are coping with their children's disease,34 defining quality of life as optimal physical and psychological wellbeing and social functioning and, as regards the dimensions associated to the child's disease, achieving emotional stability and satisfaction with family life. Emotional support and psychosocial wellbeing are key predictors of the quality of life of parents both at home and socially.15,35 For example, there is evidence of an association between a divorced and/or unemployed status of parents and low adherence to treatments.26,36 There are also studies that have assessed social factors, such as family social status based on the number of children in the family, the number of affected children, the educational attainment, employment status and marital status of the parents, and the need for intervention when certain factors are combined.26,37
For all of the above, psychological support and social counselling should be available through the public health care system.38,39 In our survey, most caregivers did not report receiving this type of assistance.
In conclusion, the results of our study show that the appropriate delivery of multidisciplinary treatment with a specific diet, medication and daily support services is affected by the inability of families to face its high costs and the low accessibility to these resources. These diseases have a considerable impact on the quality of life of patients, but especially of their main caregivers: nearly half of respondents perceived a very deleterious impact on their social, professional and family life. Thus, research should be conducted to assess the outcomes of health care, social and financial support programmes aimed at ill individuals and their caregivers to then address their needs.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Tejada-Ortigosa EM, Flores-Rojas K, Moreno-Quintana L, Muñoz-Villanueva MC, Pérez-Navero JL, Gil-Campos M. Necesidades sanitarias y socioeducativas de niños con enfermedades raras de tipo metabólico y sus familias: estudio cualitativo en un hospital de tercer nivel. An Pediatr (Barc). 2019;90:42–50.