In children, arterial ischemic stroke is a much less understood disease compared to in adults due to its lower frequency and different aetiology. However, it is also a serious disease, with a high incidence of severe and permanent sequelae that exceeds 50% of total cases.
The acute management of postnatal arterial ischaemic stroke (MNAIS) has changed drastically in recent years, chiefly on account of recanalization treatments (thrombolysis and endovascular therapies). These treatments, which used to not be recommended in childhood, are increasingly implemented in everyday clinical practice. Although the evidence from studies carried out in children is not of high quality due to their retrospective design and the small number of reported cases, they support the hypothesis that these treatments are as safe and effective as they are in adults as long as appropriate eligibility criteria are applied and they are used within a certain time from the onset of symptoms (therapeutic window).
This article reviews the MNAIS based on the current scientific evidence.
Since the efficacy of these treatments is highly dependent on their early initiation, a paediatric stroke code needs to be in place as an extension of the stroke code applied to adults. It has started to be introduced in Spain since 2019, although there are still large areas of the country where it has yet to be applied.
El ictus arterial isquémico infantil es una patología mucho menos conocida que en adultos debido a su menor frecuencia y a su diferente etiología. Sin embargo, es también una patología grave con una alta incidencia de secuelas severas y perennes, que sobrepasan el 50% de los casos.
El manejo agudo del ictus arterial isquémico postnatal (IAIPP) ha cambiado drásticamente en los últimos años, fundamentalmente en lo referente a los tratamientos de recanalización (trombólisis y terapias endovasculares). Estos tratamientos, que antes no se recomendaban en la edad infantil, se están afianzando cada vez más en la práctica diaria. Aunque los estudios realizados en niños no tienen un grado de evidencia alto por ser retrospectivos y porque el número de casos es bajo, soportan la idea de que dichos tratamientos son igual de seguros y eficaces que en los adultos siempre que se realicen con unos criterios de inclusión y exclusión determinados y dentro de un tiempo determinado desde el inicio de los síntomas (ventana terapéutica).
En este artículo se revisa, a la luz de los conocimientos actuales, el manejo agudo del ictus arterial isquémico pediátrico postnatal (IAIPP).
Debido a que la eficacia de estos tratamientos está íntimamente ligada al inicio precoz de los mismos es necesaria la existencia de un código ictus infantil como ampliación del código ictus que se aplica a los adultos. Ha empezado a implantarse en España desde el año 2019 aunque todavía hay importantes zonas del país donde aún no se aplica.
The incidence of stroke is lower in children compared to adults (2–13 cases vs. 150–200 cases per 100 000 population per year).1 The incidence of postnatal paediatric ischaemic stroke (PNAIS) in individuals aged less than 16 year is 1.60 cases per 100 000 population per year.2
However, it should not be considered a rare disease, and its effects can be devastating: it is one of the 10 leading causes of death in children, and the incidence of permanent neurologic sequelae ranges from 50% to 60% in the case of ischaemic stroke and 33% to 50% in the case of haemorrhagic stroke, with a substantial social and economic burden that it greater compared to adult stroke, as the life expectancy of paediatric patients is greater.1
There are different possible causes of stroke in children, and its aetiology differs compare to stroke in adults (Table 1), although in adolescents, the use of illicit substances and tobacco can play a role in its development.
Most frequent causes of postnatal ischaemic stroke.
| Congenital or acquired heart disease | Stroke is usually secondary to embolism. It frequently develops after surgery and in children who carry ventricular assist devices or in the context of infection. |
|---|---|
| Focal cerebral arteriopathy (FCA) | Segmental arterial narrowing in the anterior territory, commonly involving the distal carotid artery, proximal middle cerebral artery (MCA) or anterior cerebral artery (ACA). It is classified as inflammatory subtype (FCA-i), arterial dissection subtype (FCA-d) and FCA not otherwise specified. The first two subtypes probably represent a spectrum of disease. |
| Moyamoya disease and syndrome | Stenosis or occlusion of the supraclinoid segment of the internal carotid artery or segments A1 of the ACA or M1 of the MCA, typically bilateral (although bilateral involvement may not yet have developed at the time of stroke), with an anomalous collateral circulation at the base of the brain near the occluded or stenotic regions with a characteristic angiographic appearance resembling a puff of smoke (“moya moya” in Japanese). It may be isolated (in some cases, with a genetic aetiology), or secondary to certain diseases (neurofibromatosis, sickle cell anaemia, radiation therapy or Down syndrome). |
| Arterial dissection | It results from a tear in the artery wall that allows the passage of blood between the tunica intima and tunica media, dissecting them and forming an intramural haematoma, resulting in narrowing of arterial the lumen and reduced blood flow. It is classified as cervical (carotid or vertebral) or intracranial. It may result from trauma (in many cases, apparently mild trauma), but in other cases it develops spontaneously. The risk of dissection is greater in children with collagen vascular disease or elastin disorders (Marfan syndrome, Loeys-Dietz syndrome, arterial tortuosity syndrome). |
| Sickle cell anaemia | The main mechanisms are the adherence of red blood cells to the vascular endothelium and haemolysis, resulting in a hypercoagulable state, an increase in vasomotor tone and eventually thrombosis. May cause clinically significant or silent stroke, cerebral haemorrhage and Moyamoya syndrome. |
| Other genetic diseases | Thrombophilia risk factors (they generally facilitate the development of stroke, but are not pathogenic in themselves)Errors of metabolism: organic aciduria, mitochondrial disorders (MELAS), changes in ACTA2Change in NOTCH3 (CADASIL)Change in ABCC6 (elastic pseudoxanthoma)Change in COL4A and related diseasesChange in JAG1 (Alagille syndrome)Change in ATP7A (Menkes disease)Change in SLC2A10 (arterial tortuosity syndrome)Change in GLA (Fabry disease)Change in ELN (Williams syndrome)Change in NF1 (Neurofibromatosis)Change in TSC1 and TSC2 (tuberous sclerosis)Fibromuscular dysplasiaPHACE syndromeOther specific genetic disorders |
ACA, anterior cerebral artery; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; FCA, focal cerebral arteriopathy MCA, middle cerebral artery; MELAS, mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes; PHACE, posterior fossa defects, haemangioma, arterial anomalies, coarctation of the aorta and cardiac defects, eye abnormalities.
In the past 20 years, the prognosis of stroke in adults, especially in the case of ischaemic stroke, has improved substantially thanks to the introduction of recanalization treatments, the creation of the stroke code and the institution of stroke units. But these treatments have only been applied in children for a short time, and the paediatric stroke code was only introduced in Spain (in Madrid) 3 years ago.3
In consequence, we believed it would be useful to review and provide up-to-date information on the treatment of postnatal paediatric ischaemic stroke in the current article.
Pathophysiology of ischaemic strokeWhen an artery in the brain becomes obstructed, there is a decrease in cerebral blood flow that results in reduced levels of ATP, oxygen and glucose. This in turn results in decreased energy production and the development of a cascade of processes (metabolic cascade) with changes in the Na+/K+ ATPase pump, accumulation of neurotransmitters and other amines, a toxic increase in free radicals, calcium, water and hydrogen ions. There is also a significant inflammatory response. All of it leads to cell death. In the area directly affected by vascular obstruction, or ischaemic core, cell death occurs rapidly and is all but irreversible. In the surrounding tissue, or penumbra, cells remain intact at first, but if flow is not restored within a few hours, they will also suffer the consequences of the described metabolic cascade.1
Assessment and initial management of PNAISWhen a child presents to the emergency department with suspected stroke:
- -
Take a brief history including the time of onset and the previous degree of neurologic disability. The latter should be assessed by means of the modified Rankin scale (Table 2). The scale yields a score between 0 (asymptomatic) and 6 (death). It is also used to assess neurologic disability after stroke.
Table 2.Modified Rankin scale.
Level Category Description 0 No symptoms 1 No significant disability Able to carry out all usual duties and activities without limitations 2 Slight disability Unable to carry out all previous activities, but able to look after own affairs without assistance 3 Moderate disability Requiring some help, but able to walk without assistance 4 Moderately severe disability Unable to walk or attend to bodily functions without assistance of another individual, needing continuous supervision. 5 Severe disability Totally dependent. Requiring constant care. 6 Expired - -
Perform a clinical assessment. The National Institute of Health Stroke Score (NIHSS) can be used for the neurologic evaluation to guide decision-making. It comprises 14 items. Based on the score, the patient is considered to have no symptoms of stroke (0 points), mild stroke (1–4 points), moderate stroke (5–15 points), moderate/severe stroke (15–20) or severe stroke (>20). This scale has been adapted and validated for use in paediatric patients aged 4 months to 18 years (pedNIHSS).
- -
Carry out a basic blood panel including blood glucose measurement on an urgent basis.
Their purpose is to maintain and improve the viability of the penumbra and blood flow through the collateral circulation. Neuroprotection should be initiated as soon as possible, without awaiting confirmation of stroke.
It comprises:
- 1)
Absolute bed rest. There is still disagreement as to whether the head of the patient should be flat or elevated at 30º (theoretically, cerebral blood flow would be greater with the former). A recent meta-analysis in adults did not yield clear results as to whether one position is superior to the other.4 In intracranial haemorrhage is suspected, it is better to keep the head elevated because it helps decrease intracranial pressure.
- 2)
Nil per os at the outset (usually 24 h). The ability to swallow normally must be verified before initiating oral feeding.
- 3)
Fever control. Fever increases cerebral metabolism, which promotes the metabolic cascade.
- 4)
Maintain normal oxygenation. There is no evidence that supplemental oxygen improves ischaemic stroke. Therefore, oxygen should only be administered if the level of consciousness is low or the oxygen saturation drops below 95%.
- 5)
Maintain normal blood glucose levels to prevent anaerobic glycolysis. Do not administer intravenous glucose solution in patients 2 years or older unless there is evidence of hypoglycaemia.
- 6)
Urgent administration of antiepileptic drugs if the patient develops seizures.
- 7)
Treat dehydration.
- 8)
Consider the need of blood pressure (BP) monitoring. This is a controversial subject:
The increase in blood flow resulting from the raised blood pressure can be detrimental, as it promotes the described metabolic cascade and the development of bleeding.
On the other hand, an increase in blood flow is beneficial because it improves the blood supply to the penumbra through the collateral circulation.
Lastly, it must be taken into account that stress and anxiety may produce a transient increase in blood pressure.
Thus, hypertension should only be treated in carefully selected cases.
- 9)
There is evidence that iron-deficiency anaemia promotes the development of stroke in previously healthy children. Therefore, iron deficiency should be treated if the haemoglobin concentration is less than 10 g/dL.
In children, the diagnosis of ischaemic stroke requires radiological confirmation, contrary to adults, in whom a compatible clinical presentation and absence of bleeding on computed tomography (CT), even if there are no signs of stroke, suffice to start recanalization treatments. The reason is that there are many diseases that mimic the presentation of stroke in children, and several of them are more frequent than stroke. Computed tomography is the most widely available technique in emergency care and is sufficient for diagnosis in the case of haemorrhagic stroke. However, in cases of ischaemic stroke, the CT scan is frequently normal in the first few hours, so normal CT findings do not rule out the diagnosis.
Magnetic resonance imaging (MRI) is the gold standard in the paediatric population. In addition to not exposing the patient to ionising radiation, it allows diagnosis as soon as half hour after the onset of symptoms. When it is not available, CT with CT angiography may be used instead.
The assessment requires a specific MRI protocol.5 In our hospital, the following sequences are used in imaging in the order listed below:
- •
Diffusion-weighted imaging (DWI). Detects ischaemic stroke in 25 s.
- •
Fluid attenuated inversion recovery (FLAIR)
- •
Three-dimensional time-of-flight (3D-TOF) scan of the circle of Willis (MR angiography).
- •
Susceptibility weighted imaging (SWI) to assess for the presence of deoxyhaemoglobin, vessels with reduced flow and haemosiderin (signs of haemorrhage). If the presence of ischaemic stroke is confirmed, a 2D-TOF scan of the supra-aortic arches is also performed for a more thorough assessment of the entire vascular tree. For assessment of arteriopathy, especially if a large vessel is involved, specific intracranial MRI sequences may be used to determine the state of vascular walls (vessel wall imaging [VWI]).
To determine the extension of the stroke and therefor consider different treatment options, we use the Alberta Stroke Program Early CT Score (ASPECTS) (Fig. 1).
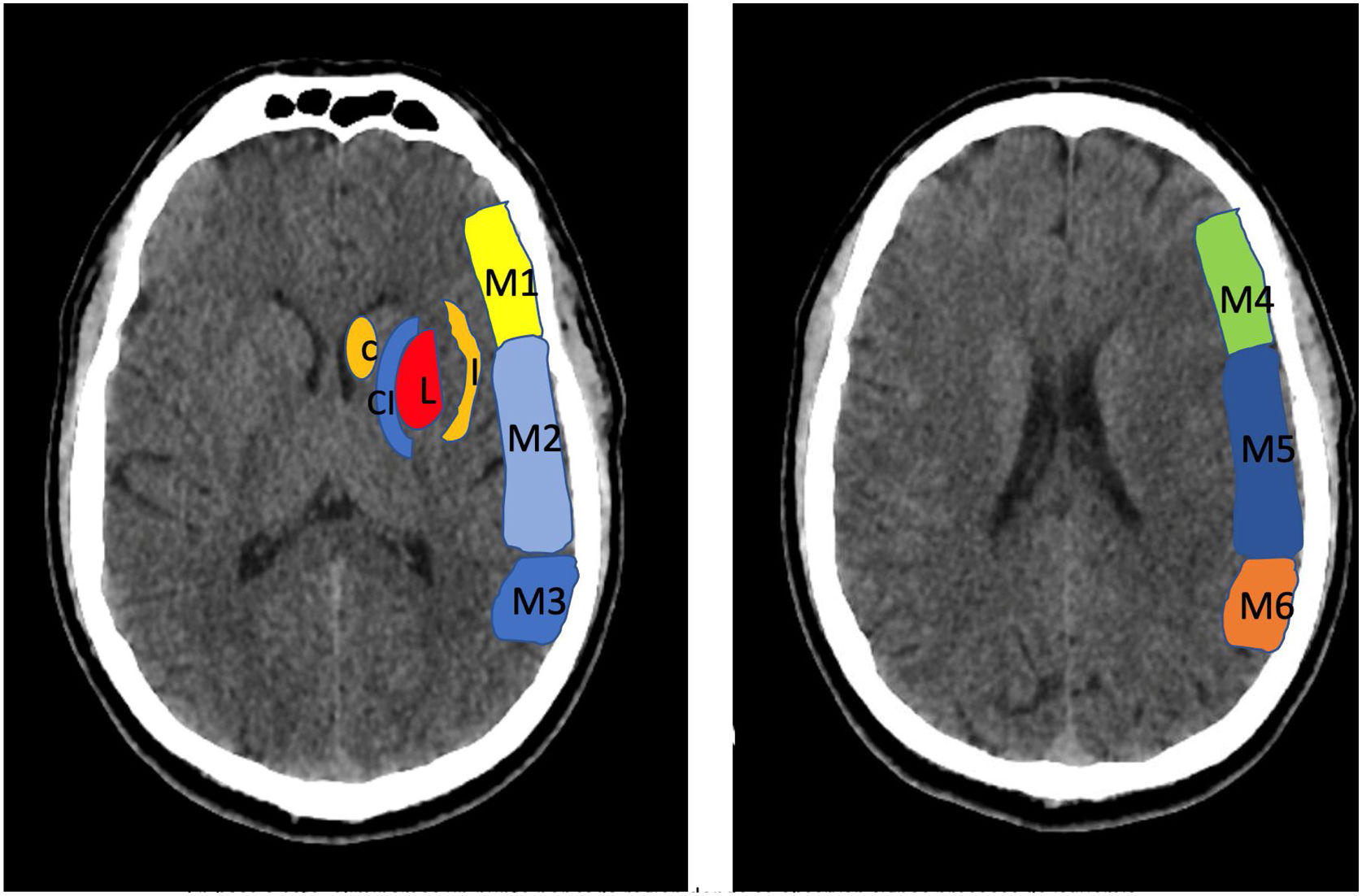
Alberta Stroke Programme Early CT Score (ASPECTS). It is used for the interpretation of a CT or MRI scan (diffusion sequence) in ischaemic stroke involving the anterior circulation. Two axial planes are analysed, the first one at the level of the thalamus and basal ganglia (plane A), and the second at the corona radiata level, in which the basal ganglia are not visible (plane B). The territory of the middle cerebral artery (MCA) in these 2 planes has been divided into 10 regions, each of which is assessed at 1 point. These regions are: L: lentiform nucleus (putamen); C: caudate; IC: internal capsule; I: insular ribbon; M1: Anterior MCA cortex; M2: MCA cortex lateral to insular ribbon; M3: posterior MCA cortex; M4, M5, M6: anterior, lateral and posterior territory of the MCA, approximately 2 cm superior to M1, M2 and M3, respectively. Based on this analysis, one point is deducted from the initial 10 for each region exhibiting early signs of ischaemia (hypodensity or local mass effect). Scores of 7 points or less are associated with poor functional recovery and high morbidity and mortality, with a higher risk of intraparenchymal haemorrhage. A score of 10 corresponds to a normal CT scan.
Computed tomography and MRI can also be used to assess the benefits and risks of recanalization treatments past the therapeutic window, which we will discuss further down.
Recanalization treatmentsIntravenous thrombolytic treatmentOnce the study published in 1995 by the National Institute of Neurological Disorders (NINDS) demonstrated the usefulness of intravenous (IV) treatment with recombinant tissue-plasminogen activator (rtPA, alteplase)6 and the European Cooperative Acute Stroke Study (ECASS)7 showed that the efficacy and safety were greater if it was administered within 4.5 h of onset, this treatment started to be used routinely in adults.
At first, paediatric stroke management guidelines did not recommend its use in children. However, the publication of several cases of paediatric stroke in which intravenous thrombolytic treatment was safe and effective motivated the development of a prospective study, the Thrombolysis in Pediatric Stroke (TIPS) trial,8 to establish the safety, appropriate dosage and feasibility of treatment with IV alteplase in children. Unfortunately, the trial was closed before completion for lack of recruitment. However, more recently, the TIPS Extended Results (TIPSTER) study,9 which analysed the cases of 26 children with stroke who had received IV thrombolytic treatment in TIPS study sites, demonstrated that the treatment was well tolerated. None of the patients developed symptomatic bleeding. Later studies have also demonstrated is tolerability.10,11
Some of the more recent paediatric guidelines contemplate the possibility of using it as long as the presence of arterial obstruction has been confirmed. However, the recent studies we just cited9–11 only required the presence of stroke with features suggestive of vascular obstruction to administer the treatment. Table 3 presents their inclusion and exclusion criteria, which were based on adult stroke protocols.
Inclusion and exclusion criteria for thrombolytic treatment.
| Inclusion criteria. Must meet all the following: |
|---|
| Age ≥2 years |
| Time between onset of symptoms and administration of thrombolytics <4.5 h (time of onset must be known). |
| Initial Rankin ≤2. Consider if >2 depending on quality of life and social or family support |
| Clinical and radiological features of ischaemic stroke with a NIHSS ≥4 (consider patients with NIHSS <4 in case of extremity paralysis, severe aphasia or hemianopsia |
| No intracranial haemorrhage |
| Informed consent of parents. In special circumstances, it can be provided by a bystander or other relatives or caregivers |
| Exclusion criteria. Any one of these criteria is a contraindication: |
|---|
| Age <2 years |
| Initial Rankin >2 with poor quality of life or without social/family support |
| More than 4.5 h from onset of ischaemic stroke, unknown time of onset or wake-up stroke |
| Evidence of intracranial haemorrhage on CT |
| Clinical suspicion or signs of subarachnoid haemorrhage |
| Previous history of intracranial haemorrhage or AVM or aneurism or intracranial tumour |
| Minor symptoms or marked improvement without disabling impairment before infusion. Mild deficit (PedNIHSS <4) at initiation of tPA infusion. (UK PedNIHSS 4−24, TIPS PedNIHSS 4−24). Consider treatment in patients with a NIHSS <4 and extremity paralysis, severe aphasia or hemianopsia |
| PedNIHSS >25 |
| SBP persistently more than 15% above the 95th percentile for age (sitting or lying down) |
| Pronounced and extensive hypodensity on CT |
| Previous stroke, severe head trauma or intracranial surgery in the past 3 months |
| Cancer with life expectancy < 6 months or with increased risk of haemorrhage |
| Stroke secondary to bacterial endocarditis, sickle-cell anaemia, meningitis, embolism (bone marrow, air, fat), Moyamoya disease |
| Gastrointestinal or genitourinary haemorrhage <21 days, gastrointestinal cancer |
| Significant bleeding diathesis: mild platelet function disorders, mild von Willebrand disease or other mild bleeding diatheses are NOT excluded. |
| Major surgery or brain biopsy in the past 10 days |
| Arterial puncture in a non-compressible site or lumbar puncture in the past 7 days. Cardiac catheterization through a compressible artery is NOT a contraindication. |
| Previous diagnosis of primary vasculitis of the CNS or secondary arteritis |
| Severe head trauma in the past 3 months (watch for trauma associated with stroke) |
| LMWH at anticoagulant dose in the past 24 h |
| Cerebral arterial dissection |
| If the patient is taking oral anticoagulants, if INR > 1.4 (1.7 in adults). If the patient has taken heparin in the past 4 h, the aPTT must be normal |
| Consumption of dabigatran or apixaban or rivaroxaban in the past 48 h (unless antidote is available) |
| Platelets <100 000 (no need to wait without suspicion), PT > 15 s (INR > 1.4) or PTT above upper limit of normal for age. |
| Pregnant or postpartum < 14 days post birth, consult with gynaecologist re: eligibility |
| Stroke in patient with pericarditis or heart attack |
In adults, studies have been conducted that provided this treatment to patients past the therapeutic window, always having determined the volume of ischaemic core and penumbra tissue, as we will see later,12,13 but there is no evidence on the subject in children.
Endovascular therapyIts purpose is to remove the clot, for which a microcatheter is inserted in the femoral or radial artery and passed to the occluded vessel. Once there, one option is to infuse drugs to dissolve the clot, but at present the preferred option is to deliver a device to extract the clot (mechanical thrombectomy).
Several large trials in adults14–20 and one meta-analysis21 have demonstrated that endovascular treatment is beneficial when conducted within 6 h of the occlusion of the intracranial internal carotid or the M1 segment of the middle cerebral artery. The meta-analysis found a risk of symptomatic intracranial haemorrhage of 4.4% but no evidence of an increase in mortality in the intervention group compared to the control group.
Endovascular therapies are not a contraindication for thrombolytic treatment and it is very common to use both (in the aforementioned studies, 5 out of 6 patients who underwent endovascular treatment had previously received thrombolytic drugs).
There are fewer sources on the use of endovascular therapies for management of posterior circulation stroke, chiefly secondary to obstruction of the basilar artery. However, two trials conducted in adults have been published recently22,23 that confirm the efficacy and safety of thrombectomy in these cases.
As occurred with thrombolytic treatment, paediatric stroke management guidelines did not initially recommend the use of endovascular therapies due to a lack of evidence. However, due to the publication of numerous case reports evincing a favourable response and few complications, more recent guidelines24–26 no longer discourage the use of endovascular treatment and even state that it may be justified in patients with a pedNIHSS of 6 or greater, with obstruction of a great vessel, in older children, within 6 h of onset and with the agreement of the paediatric neurologist and the interventional neuroradiologist.
The multicentre observational Save ChildS Study, published in 2020,27 and a recent meta-analysis28 found that the safety of endovascular treatment in children was not inferior to the safety observed in adults.
In consequence, there is widespread consensus that endovascular therapies can be used in children with stroke applying inclusion and exclusion criteria that are essentially the same as those applied in adults (Table 4).
Inclusion and exclusion criteria for mechanical thrombectomy.
| Inclusion criteria. Must meet all the following: |
|---|
| Pre-stroke modified Rankin scale ≤2 |
| NIHSS ≥6 |
| ASPECTS score (simple head CT) ≥6 |
| Isolated occlusion of the intracranial internal carotid artery or proximal middle cerebral artery (M1 segment) |
| Time elapsed from onset of stroke <6 h. In patients with onset > 6 h before or posterior circulation involvement, determine eligibility on a case-by-case basis. |
| Age <2 years (it is not an absolute requirement, can be reconsidered) |
| Obtain specific informed consent from patient or family |
| Exclusion criteria. Any one of these criteria is a contraindication: |
|---|
| Concomitant disease that is severe or has a low short-term survival |
| Acute cerebral haemorrhage or clear haemorrhagic transformation in the same vascular territory. Suspected HSA |
| In posterior circulation stroke, any of the following: clinical signs indicative of irreversible extensive damage (prolonged coma or complete and persistent loss of brainstem reflexes) or neuroimaging evidence of extensive damage to the brainstem |
| Prolonged coma (>6 h) or complete and persistent loss of brainstem reflexes |
| Platelet count <60 000 or significant coagulation changes |
aPTT, activated partial thromboplastin time; ASPECTS, Alberta Stroke Program Early CT Score; AV, Marteriovenous malformation; CNS, central nervous system; CT, computed tomography; INR, international normalised ratio; LMWH, low molecular weight heparin; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; PedNIHSS, paediatric NIHSS; PT, prothrombin time; PTT, partial thromboplastin time; SBP, systolic blood pressure; tPA, tissue plasminogen activator.
As was the case of thrombolytic treatment, studies have been conducted to determine the usefulness of endovascular treatments past the therapeutic window.
Determining whether endovascular treatment can beneficial past the window requires determining the difference in volume between the penumbra (ischaemic area that can be recovered) and the infarcted area (stroke core). This difference is known as the mismatch ratio. If the core is small and the penumbra is large, that is, the mismatch ratio is substantial, there is a broad area that could be rescued, so it would be appropriate to perform thrombectomy past 6 h from onset. Several methods are available to calculate the volume of the core and the penumbra. The most suitable are perfusion imaging techniques, either perfusion CT or perfusion MRI.
Two large adult trials29,30 assessed the use of endovascular treatment in patients with ischaemic stroke performed 6–24 h and 6–16 h after onset. Both studies required a significant mismatch to consider the patient eligible for the procedure. Both found that endovascular thrombectomy was effective and safe.
In children, an extension of the Childs Study31 analysed 20 patients who underwent thrombectomy more than 6 h after stroke onset after confirming the presence of a significant mismatch. The outcomes were excellent, with a mean Rankin score of 2 at 3 months post intervention and of 0 at 24 months post intervention, with very mild complications. Another study in 38 patients32 found favourable outcomes in 84.2% of the sample (NIHSS ≤4; modified Rankin scale ≤1).
An aspect that remains controversial is the age from which endovascular therapies can be used. The Save Childs Study suggested that outcomes are less favourable in children aged less than 6 years. However, at present, given the significant advances made in the devices used by neuroradiologists, we believe that rigid age thresholds should not be applied. In fact, several published case reports have described positive outcomes of thrombectomy in infants (one in Spain) and newborns.33–36 Nevertheless, clinicians and neuroradiologists must assess each case individually to determine the appropriateness of these interventions.
Acute treatment in patients with cardiogenic embolic strokeA majority of cerebral embolisms occur in children with heart disease, especially congenital heart defects.
The indication of recanalization, fibrinolytic and endovascular therapies in these therapies can be determined applying the previously discussed inclusion and exclusion criteria.
Acute treatment in patients with arteriopathyIn patients with arteriopathy, which entails inflammation or injury of vascular walls, the use of thrombolytic treatment or thrombectomy is even more controversial due to the increased risk of dissection, vasospasm and rupture.
Vessel wall imaging (VWI) MRI may be useful to assess the condition of the vessel walls and guide decision-making.
Focal cerebral arteriopathyFocal cerebral arteriopathy is not a contraindication for thrombolytic therapy as long as the established inclusion and exclusion criteria are applied.
When it comes to mechanical thrombectomy, there is a dearth of evidence on the outcomes of patients with focal cerebral arteriopathy.
The Save ChildS Study27 included only 7 children with arteriopathy, in all likelihood for the reasons noted above. Another recent multicentre study37 on recanalization therapies for PNAIS conducted in a paediatric sample in France that included 17 children with focal cerebral arteriopathy did not find significant complications, but the frequency of arterial reocclusion or persistent stenosis greater than 50% was very high.
Thus, although performance of thrombectomy should not be ruled out by default, its appropriateness should be evaluated by neuroradiologists and clinicians on a case-by-case basis.
Intracranial artery dissectionIn this context, recanalization therapies also pose theoretical risks. Intravenous thrombolytic treatment carries a risk of intradural haematoma. In thrombectomy, there is a risk of entering the false lumen of the tear, which can result in arterial perforation.
The European Stroke Organisation (ESO) guideline38 (developed for the adult population) may be used to guide decision-making.
Moyamoya disease and syndromeTheoretically, if a patient with moyamoya experiences a stroke, neither fibrinolytic therapy nor thrombectomy are recommended because the intracranial vessels are friable and there is a high risk of haemorrhage. However, a recent systematic review39 described 10 cases of patients who underwent thrombolytic therapy, thrombectomy or both with favourable outcomes. The authors concluded that these interventions could be performed in carefully selected cases.
Sickle cell anaemiaIn this disease, the most frequent stroke mechanisms are the adherence of red blood cells to the vascular endothelium and haemolysis, resulting in a hypercoagulable state, an increase in vasomotor tone and eventually thrombosis. The standard of care in children with sickle cell anaemia who develop ischaemic stroke is, in addition to supplemental oxygen, immediate initiation of exchange transfusion if the haemoglobin (Hb) concentration decreases below 8.5 g/dL (ideally within 2 h of onset) to achieve a target Hb concentration of 10 g/dL. Exchange transfusion is preferable to simple transfusion to achieve a proportion of sickle haemoglobin (HbS) of less than 15%–20%. If the Hb concentration is greater than 8.5 g/L, exchange transfusion is preferable to reduce the risk of hyperviscosity.
In adults aged more than 18 years, the American Society of Hematology does not rule out the use of thrombolysis within 4.5 h of onset based on the applicable inclusion and exclusion criteria as long as it does not delay initiation of simple or exchange transfusion. However, the use of IV tPA is not recommended in patients aged less than 18 years (conditional recommendation due to the scarcity of the evidence in the paediatric population).40
Paediatric stroke codeAs evinced by what has been discussed this far, stroke is a medical emergency. Each minute, 1.9 million neurons are lost. This motivated the development of the stroke code (whether the stroke is ischaemic or haemorrhagic), whose purpose was to prioritise the treatment of affected patients. However, this code was not applied to patients under 18 years. The reasons for this omission were the infrequency of stroke in paediatric patients, that they appeared to recover better than adults and that recanalization treatments should not be implemented in paediatric patients age group due to the lack of evidence in this age group. As this article demonstrates, these reasons are no longer valid.
In consequence, after years of debate and negotiation, in 2019 the stroke code was finally extended to include patients under 18 years in the Community of Madrid, which was the first region in Spain to apply it as an extension of the pre-existing adult stroke code.3 The use of the paediatric stroke code is spreading to other regions, and we hope that it will soon be implemented nationwide in Spain.
There are 2 distinct phases in the stroke code: the prehospital management of stroke, from onset of symptoms to the arrival of the patient to the hospital, and the acute inpatient care phase, from arrival to hospital, including diagnosis and treatment of stroke. To optimise outcomes, we recommend adherence to the time goals detailed in Table 5.
Paediatric stroke code. Established time goals.
| From onset to arrival to hospital | Less than 120 min |
| From activation of stroke code to arrival to hospital | Less than 60 min |
| From arrival to emergency department to neuroimaging, with less than 5 min thereafter to confirm or rule out stroke | Less than 20 min |
| From performance of neuroimaging to confirmation of diagnosis | Less than 5 min |
| From onset to insertion (IV thrombolysis) less than 120 min for IV thrombolysis and less than 200 min for endovascular recanalization. | Less than 120 min |
| From arrival to hospital to insertion (IV thrombolysis) | Less than 45 min |
| From onset to endovascular recanalization | Less than 200 min |
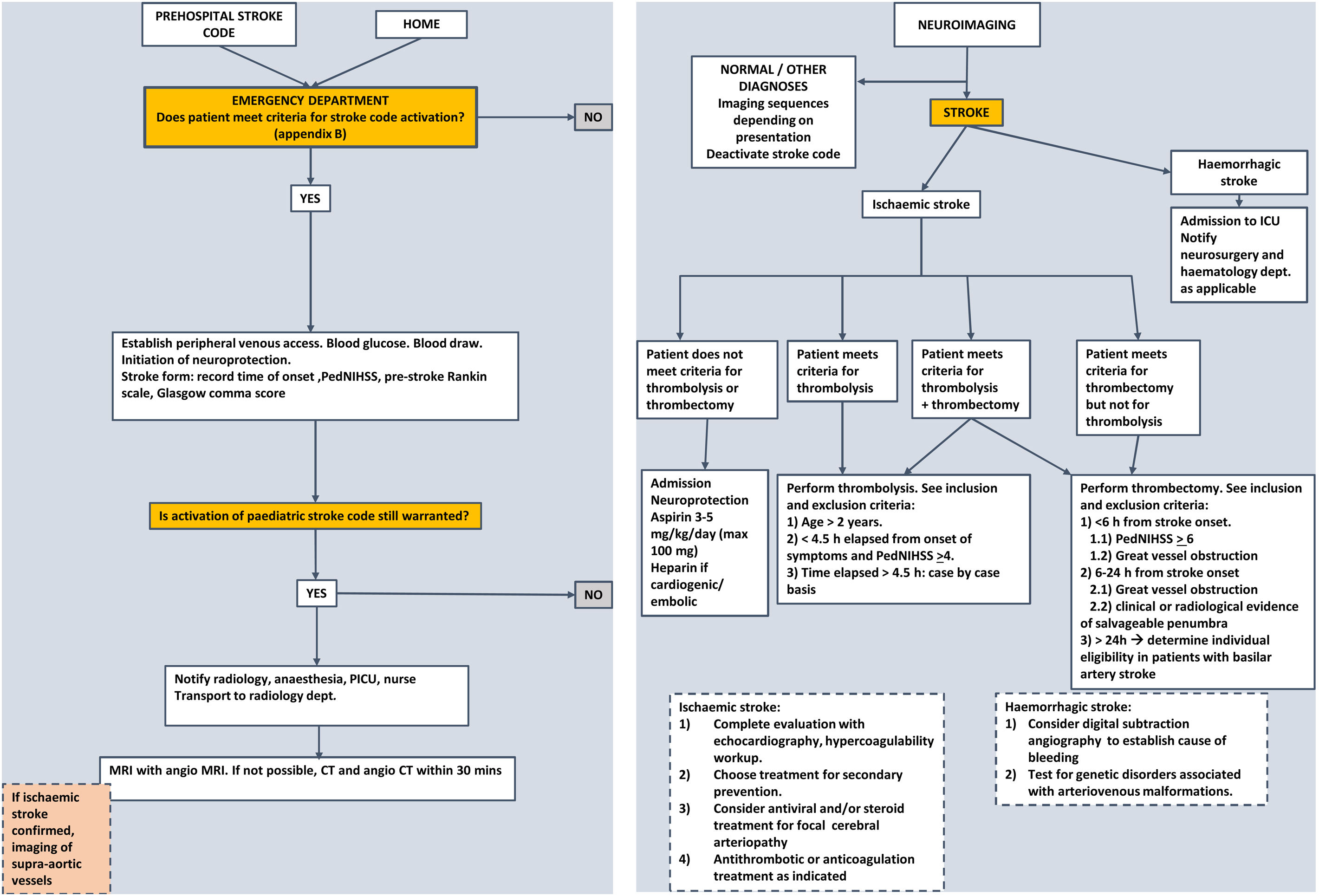
The management of paediatric ischaemic stroke has changed substantially in recent years. The nihilistic attitude toward it, compared to the treatment of stroke in adults, has no justification at present, as acute recanalization treatments can and should be used in children, albeit with certain precautions. Since these treatments are more effective the earlier they are implemented, the stroke code should be applied to any child with a compatible presentation. Management algorithms summarising the steps to take in any paediatric patient with suspected stroke, like the one presented in Fig. 2, need to be developed.
Conflict of interestsThe authors declare that they have no conflict of interest.