Although the use of ultrasound for the insertion of central catheters has proven to be cost-effective in adults, it is not known if this is the case in the neonatal population. This study compared the cost-effectiveness of ultrasound-guided umbilical venous catheterisation with conventional catheterisation in a neonatal intensive care unit of a Public University Hospital.
Patients and methodsA retrospective observational study was conducted on newborns that required an umbilical venous catheter before completing their first 24h of extra-uterine life. Two retrospective cohorts were formed, including one with ultrasound-guided catheterisation and the other with conventional catheterisation. The effectiveness was measured using 2 variables: placement of ideal position and insertion without complications. The cost of human and material resources (consumable and non-consumable), the cost-effectiveness ratio, and the incremental cost-effectiveness ratio were estimated, as well as carrying out a sensitivity analysis.
ResultsCatheter obstruction was more frequent in guided catheterisation than in conventional catheterisation (7.7% vs. 0%, P=.04) and catheter dysfunction was higher in the latter (79% vs. 3.8%, P<.0001). The cost-effectiveness ratio of the guided catheterisation was €153.9, and €484.6 for the conventional one. The incremental cost-effectiveness ratio was €45.5. The sensitivity analysis showed a €2.6 increase in the cost-effectiveness ratio of the guided catheterisation and €47 in the conventional one.
ConclusionsThe use of ultrasound to guide umbilical catheterisation is more efficient than conventional catheterisation since, despite using more economic resources, it offers greater effectiveness.
El uso de ultrasonografía para la inserción de catéteres centrales ha mostrado ser coste-efectivo en adultos; en neonatos se desconoce esta información. El objetivo del estudio fue comparar el coste-efectividad de la cateterización venosa umbilical guiada por ultrasonografía con la cateterización convencional en un servicio de cuidados intensivos neonatales de un hospital universitario y público.
Pacientes y métodosEstudio observacional retrospectivo en recién nacidos que requirieron catéter venoso umbilical antes de cumplir las primeras 24h de vida extrauterina; se conformaron 2 cohortes históricas, una con cateterización guiada por ultrasonografía y otra con cateterización convencional. La efectividad se midió con 2 variables: colocación de posición ideal e inserción sin complicaciones. Se estimó el coste de recursos humanos y materiales (fungibles y no fungibles), la razón coste-efectividad y la razón coste-efectividad incremental; y se realizó análisis de sensibilidad.
ResultadosLa obstrucción del catéter fue más frecuente en la cateterización guiada que en la convencional (7,7 vs. 0%, p=0,04) y la disfunción del catéter fue superior en esta última (79 vs. 3,8%, p<0,0001). La razón coste-efectividad de la cateterización guiada fue 153,9 euros y de la convencional 484,6 euros; la razón coste-efectividad incremental fue 45,5 euros. El análisis de sensibilidad incrementó 2,6 euros en la razón coste-efectividad de la cateterización guiada y 47 euros, en la convencional.
ConclusionesEl uso de la ultrasonografía para guiar la cateterización umbilical es más eficiente ya que, a pesar de suponer un mayor consumo de recursos económicos, ofreció una mayor efectividad.
Central venous catheters are frequently used in neonatal intensive care units, and access through the umbilical vein is widely used for delivery of medicines, parenteral nutrition and blood products and to obtain samples for laboratory testing.1,2 Umbilical venous catheterization offers the advantage of preventing the complications associated to repeated peripheral vein punctures and the associated pain.3,4 Traditionally, insertion is performed blindly after calculating the length of catheter that needs to be inserted based on equations that use external anatomical references.4–6 Ideally, the distal catheter tip should be placed at the inferior vena cava-right atrial junction, and correct placement is verified by means of an anteroposterior X-ray. In 20–30% of cases, the inserted length is insufficient or excessive, that is, the tip is found in the right atrium or the left atrium, which may lead to thrombosis or arrythmias. The catheter may also be malpositioned in the portal vein.4,7–12 On the other hand, there is evidence that ultrasound-guided umbilical venous catheterization achieves a higher success rate at the first attempt. It is also associated with a lower frequency or earlier detection of complications.4,5,13–17 Ultrasound-guided placement of central venous catheters has proven cost-effective in adults,18–21 but there are no data on this aspect for the neonatal population.
The aim of our study was to compare the cost-effectiveness of 2 approaches for umbilical venous catheterization (ultrasound-guided and conventional) by means of 2 endpoints (optimal umbilical catheter position and insertion without complications) in a public university hospital.
Materials and methodsStudy design, inclusion criteria and sample size calculationWe conducted a single-centre observational retrospective study from June to December 2017 in the neonatal intensive care unit of a tertiary care university hospital in Northeast Mexico. The sample included newborns that required elective umbilical venous catheterization within 24h of birth after performance of any necessary resuscitation and stabilisation procedures. We analysed 2 historic cohorts, one corresponding to patients that underwent ultrasound-guided catheterization and the other to patients that underwent conventional catheterization. We collected the data from patient health records. We did not set any exclusion criteria, and only eliminated cases in which records were incomplete. The sample size was large enough to calculate a minimum difference in the effectiveness of procedures of 20 percentage points with a power of 80% and a 95% level of confidence. The protocol of the study was reviewed and approved by the Research Ethics Committee of the Hospital Universitario Doctor José Eleuterio González and the School of Medicine of the de la Universidad Autónoma de Nuevo León (file number PE17-00009). The research procedures were overseen throughout the study to ensure the confidentiality of the data.
Description of catheterization proceduresUmbilical venous catheterization was carried out in adherence to clinical practice guidelines currently considered the standard of care,22 which included the use of aseptic techniques and disinfectants, preparation of a sterile field, performance of a purse string suture and cutting of the umbilical cord 2.0cm from the abdominal wall. The catheters used were made of polyurethane (Argyl®™ or Arrow®™), size 3.5–4 French in infants with weights of less than 3500g and 5 French in infants with weights of 3500g or greater. The decision regarding the type of approach used for catheter insertion rested with the neonatologist in charge of the patient, usually residents in the last year of their speciality training with a similar level of experience, who were also responsible for the performance of the procedure.
Ultrasound-guided catheterization. Once the umbilical vein was identified and the cord secured, the catheter was inserted guided by real-time doppler Colour ultrasound (Chison Medical Imaging Co. Ltd; Wuxi, Jiangsu, China) using a linear array probe (D12L40L 7–18MHz transducer) and the subcostal window, with a protocol similar to the one described by Fleming and Kim.23 Once the tip was visualised in the optimal position, the practitioner performed the purse-string suture, ensured haemostasis and secured the catheter to the abdominal wall with tape.
Conventional catheterization. After finding the umbilical vein and securing the cord, the practitioner inserted a length of catheter calculated with the formula developed by Shukla and Ferrara24 ([birth weight×3+9]/2+1cm) was introduced. The correct position of the tip was confirmed with a thoracoabdominal anteroposterior radiograph; if an excessive length had been inserted, placement was corrected by pulling the catheter back. Once placement was complete, the practitioner performed the purse-string suture, ensured haemostasis and secured the catheter to the abdominal wall with tape.
In both approaches (ultrasound-guided and conventional), a nurse assisted the resident physician. In ultrasound-guided catheterization, the ultrasound device was handled by a second physician, also a resident in neonatology, while in conventional catheterization, the procedure involved the collaboration of a radiographer in charge of the X-ray machine.
Assessment of effectivenessWe took 2 endpoints into account: (a) ideal catheter position (placement of distal catheter tip in right atrium, inferior vena cava-right atrial junction or thoracic inferior vena cava [≤1cm above the diaphragm])16 and (b) catheterization without complications, such as creation of a false tract, perforation of a blood vessel, cardiac arrhythmia or cardiac tamponade, permanent obstruction (inability to infuse substances through the catheter), malfunction (lack of blood return) or sepsis. We assessed for the presence of these complications from the start of the procedure to the time the patient was discharged. We estimated the success rate of optimal catheter tip placement by calculating the number of cases with optimal positioning/total cases for the type of procedure×100. On the other hand, we calculated the success rate of insertion without complications as the number of cases free of complications/total cases for the type of procedure×100. Subsequently, we calculated an effectiveness index that combined both success rate, similar to the one calculated in a cost-effectiveness study conducted in China.21 Expert neonatologists considered that the absence of complications should be given a greater weight compared to optimal placement, and a consensus was reached to give those factors weights of 0.65 and 0.35, respectively, so that the weighted effectiveness index was calculated as follows: (success rate of correct placement×0.35)+(success rate of placement without complications×0.65).
Assessment of costsWe estimated direct costs by calculating the costs of human resources, disposable supplies (such as gauze) and reusable supplies (ultrasound machine, X-ray machine) involved in each procedure. (a) Human resources: we determined the duration of each procedure (time elapsed from initiation of aseptic measures to catheter fixation, in minutes). After this, we calculated the cost corresponding to the time spent in the procedure based on the establish salary for the staff category of each individual involved in the procedure, information we obtained from the department of human resources of the hospital. (b) Disposable supplies: we calculated the cost of the catheters, gauzes, sterile fields and solutions documented to have been used in each procedure under study, and then multiplied the single-unit cost by the number of units used. We obtained the information on single-unit retail prices at the time of the study from the accounts payable department. (c) Reusable supplies: we used the cost-per-use estimated by the accounts payable department of the hospital for the ultrasound machine and the X-ray machine. This cost is based on the expected number of tests that will be performed before the equipment becomes obsolete. We calculated the total cost by adding the human resources costs to the material resources costs and multiplying the sum by the total number of patients that received each procedure. The currency we used in the initial calculation was the Mexican peso, but we then converted it to euro applying the exchange rate of November 1, 2017 (22.26 Mexican pesos to 1 euro).
Other variables under studyFor each patient, we also collected data on maternal age, gestational age in weeks at birth, neonatal anthropometric measures (weight, length, head circumference, chest circumference, abdominal girth), 1-min and 5-min Apgar scores, haemoglobin concentration and blood gas parameters (oxygen saturation, pH, partial pressure of carbon dioxide and partial pressure of oxygen). We also collected information on the type of delivery (vaginal/caesarean section), complicated delivery or emergency birth.
Statistical methods. We calculated the cost-effectiveness ratio by dividing the total cost by the effectiveness index; a low ratio was indicative of higher cost-effectiveness and a high ratio of a lower cost-effectiveness. We also calculated the incremental cost-effectiveness ratio per additional unit of benefit25 by dividing the difference in costs by the difference in effectiveness between procedure A (ultrasound-guided catheterization) and procedure B (conventional catheterization): [Cost A−Cost B]/[Effectiveness A−Effectiveness B]. This indicator represents the actual cost of each unit of effectiveness gained with procedure A. Lastly, we conducted a probabilistic sensitivity analysis using Monte Carlo simulation (10000 iterations) to assess the potential effect of randomly and simultaneously changing the total cost and the effectiveness index within the limits established by the 95% confidence intervals to then examine the effects that would emerge in the final results.26 We conducted a descriptive analysis estimating means and standard deviations for quantitative variables and proportions for qualitative variables. We used the Student's t test or the Mann–Whitney U test to compare quantitative variables and the chi square test or Fisher exact test to compare qualitative variables. We defined statistical significance as a P-value of less than 0.05.
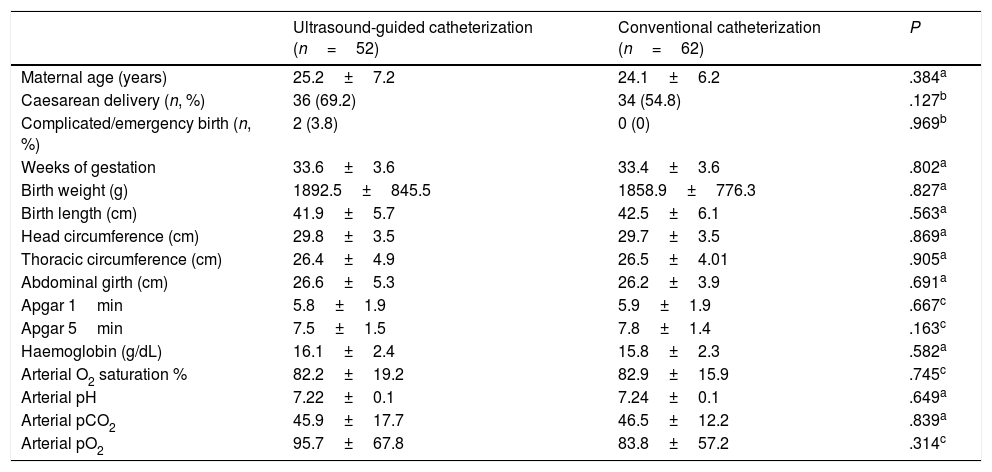
ResultsThe study included a total of 116 patients, 53 that underwent ultrasound-guided catheterization and 63 that underwent conventional catheterization, but we eliminated 2 patients from the analysis (1 per group) due to incomplete data. The mean gestational age was 33.5±3.6 weeks, the mean birth weight was 1874±805g, and the mean length 42.2±5.9cm. The patients in the two groups were equivalent in terms of clinical, anthropometric and haematologic parameters (Table 1).
Clinical, anthropometric and haematologic parameters in newborns that underwent umbilical venous catheterization.
| Ultrasound-guided catheterization (n=52) | Conventional catheterization (n=62) | P | |
|---|---|---|---|
| Maternal age (years) | 25.2±7.2 | 24.1±6.2 | .384a |
| Caesarean delivery (n, %) | 36 (69.2) | 34 (54.8) | .127b |
| Complicated/emergency birth (n, %) | 2 (3.8) | 0 (0) | .969b |
| Weeks of gestation | 33.6±3.6 | 33.4±3.6 | .802a |
| Birth weight (g) | 1892.5±845.5 | 1858.9±776.3 | .827a |
| Birth length (cm) | 41.9±5.7 | 42.5±6.1 | .563a |
| Head circumference (cm) | 29.8±3.5 | 29.7±3.5 | .869a |
| Thoracic circumference (cm) | 26.4±4.9 | 26.5±4.01 | .905a |
| Abdominal girth (cm) | 26.6±5.3 | 26.2±3.9 | .691a |
| Apgar 1min | 5.8±1.9 | 5.9±1.9 | .667c |
| Apgar 5min | 7.5±1.5 | 7.8±1.4 | .163c |
| Haemoglobin (g/dL) | 16.1±2.4 | 15.8±2.3 | .582a |
| Arterial O2 saturation % | 82.2±19.2 | 82.9±15.9 | .745c |
| Arterial pH | 7.22±0.1 | 7.24±0.1 | .649a |
| Arterial pCO2 | 45.9±17.7 | 46.5±12.2 | .839a |
| Arterial pO2 | 95.7±67.8 | 83.8±57.2 | .314c |
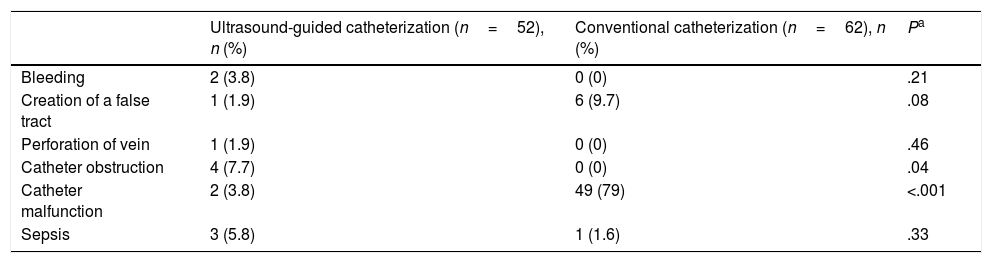
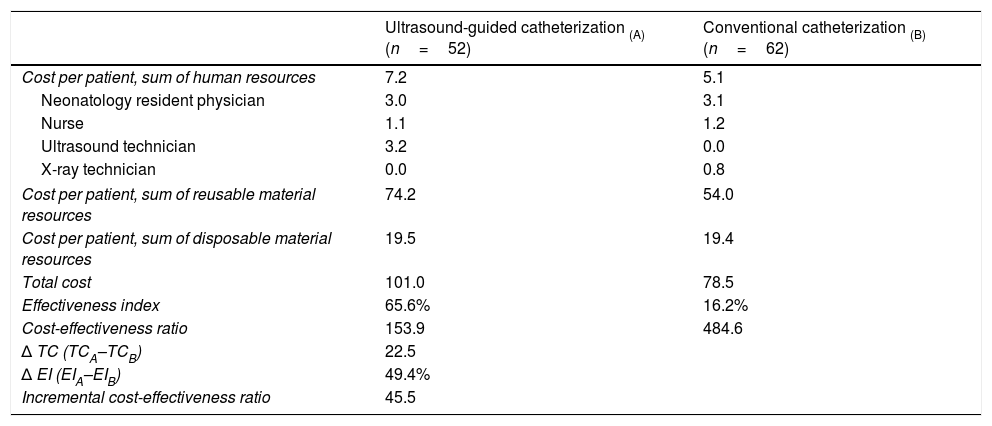
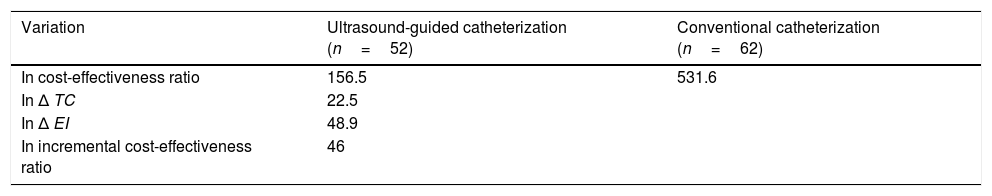
The approximate duration of ultrasound-guided catheterization was 42.2±17.8min compared to 44.2±18min in conventional catheterization (P=.56). The rates of success of optimal catheter tip placement were 48.1% for ultrasound-guided catheterization and 19.4% for conventional catheterization (P<.002). The rates of catheterization without complications were 75% and 14.5%, respectively (P<.0001). The most frequent complications were catheter obstruction in the ultrasound-guided catheterization group and catheter malposition in the conventional catheterization group (Table 2). Table 3 details the cost and effectiveness index for each type of procedure; the difference found in total cost was due to the cost of reusable equipment, which was higher in ultrasound-guided catheterization. When it came to effectiveness, the cost-effectiveness ratio was lower in the ultrasound-guided group. The sensitivity analysis found an increase in the cost-effectiveness ratio of 2.6 euro per ultrasound-guided catheterization, of 47 euro per conventional catheterization and of 0.5 euro in the incremental cost-effectiveness ratio (Table 4).
Type of complications observed during or after umbilical venous catheterization.
| Ultrasound-guided catheterization (n=52), n (%) | Conventional catheterization (n=62), n (%) | Pa | |
|---|---|---|---|
| Bleeding | 2 (3.8) | 0 (0) | .21 |
| Creation of a false tract | 1 (1.9) | 6 (9.7) | .08 |
| Perforation of vein | 1 (1.9) | 0 (0) | .46 |
| Catheter obstruction | 4 (7.7) | 0 (0) | .04 |
| Catheter malfunction | 2 (3.8) | 49 (79) | <.001 |
| Sepsis | 3 (5.8) | 1 (1.6) | .33 |
Cost (in euro) and effectiveness of umbilical venous catheterization.
| Ultrasound-guided catheterization (A) (n=52) | Conventional catheterization (B) (n=62) | |
|---|---|---|
| Cost per patient, sum of human resources | 7.2 | 5.1 |
| Neonatology resident physician | 3.0 | 3.1 |
| Nurse | 1.1 | 1.2 |
| Ultrasound technician | 3.2 | 0.0 |
| X-ray technician | 0.0 | 0.8 |
| Cost per patient, sum of reusable material resources | 74.2 | 54.0 |
| Cost per patient, sum of disposable material resources | 19.5 | 19.4 |
| Total cost | 101.0 | 78.5 |
| Effectiveness index | 65.6% | 16.2% |
| Cost-effectiveness ratio | 153.9 | 484.6 |
| Δ TC (TCA–TCB) | 22.5 | |
| Δ EI (EIA–EIB) | 49.4% | |
| Incremental cost-effectiveness ratio | 45.5 | |
EI, effectiveness index; TC, total cost.
Sensitivity analysis, impact of variationsa in cost (in euro) and effectiveness.
| Variation | Ultrasound-guided catheterization (n=52) | Conventional catheterization (n=62) |
|---|---|---|
| In cost-effectiveness ratio | 156.5 | 531.6 |
| In Δ TC | 22.5 | |
| In Δ EI | 48.9 | |
| In incremental cost-effectiveness ratio | 46 |
EI, effectiveness index; TC, total cost; Δ EI, (EIA–EIB); Δ TC, (TCA–TCB).
This study analysed the cost-effectiveness ratio of including ultrasound monitoring in the procedure of catheterization of the umbilical vein in comparison to the conventional procedure, as to date there were no studies on the subject in the literature. Our study found that the ultrasound-guided procedure is more effective.
When it comes to any catheterization procedure, it is important to consider the time it will take to achieve optimal positioning of the catheter tip. The duration of the procedure is an important factor because the longer it takes, the more handling there is and therefore the more the risk of complications increases. In our study, the mean duration of the catheterization procedure was less than 45min for both approaches, which was considerably shorter compared to the mean found by Fleming and Kim,23 who reported durations of 75±25min for ultrasound-guided catheterization and 139±49min for conventional catheterization. As for the type of complications, we ought to highlight the frequency of catheter malfunction with the conventional procedure, as there was no spontaneous blood return in 8 out of every 10 insertions, a mechanical complication that indicates occlusion of the catheter tip by a vessel wall or a fibrin sheath. Creation of a false track also tended to be more frequent in this group, although this result just felt short of being statistically significant. These 2 complications can be explained due to the nearly blind positioning of the catheter. This very low effectiveness stands in stark contrast with the ultrasound-guided approach, which is surprising given that the calculation of the length of catheter to be inserted in the conventional procedure is made with formulas based on external anatomical references.24 In this regard, some authors have reported low rates of successful insertion using formulas that range from 24% to 55.7%.6,27 In addition, the lack of blood return, when due to fibrin, is indicative of a state preceding total obstruction, and while the latter occurred more frequently in newborns that underwent ultrasound-guided catheterization, when combined with catheter malfunction, the frequency was higher in newborns that underwent conventional catheterization. We are unable to explain the higher frequency of catheter obstruction in the ultrasound-guided group. Future studies should prospectively assess those factors involved in the progression from umbilical venous catheter malfunction to obstruction.
In terms of costs, the total cost differed mainly on account of the cost of the equipment and the participation of staff to handle the ultrasound machine, but the cost-effectiveness ratio was better for the ultrasound-guided procedure due to the lower frequency of catheter malposition and of catheter-related complications. Cost-effectiveness studies on ultrasound-guided central catheter insertion in adults have found similar results.20,21,28 The use of ultrasonography during umbilical venous catheterization offers the advantage of guiding the catheter and repositioning the tip in real time. It also requires minimum handling of the newborn, allows detection of central catheter migration and reduces the exposure to radiation involved in the use of conventional radiography.29 The incremental cost per unit of effectiveness in ultrasound-guided catheterization was of approximately 50 euro, and the estimated sensitivity taking into account possible variations in cost and effectiveness diverged little from the initial estimates, which would support recommending the use of the ultrasound-guided procedure. However, these findings must be interpreted with caution. In this study, the procedures were performed by physicians-in-training in the last year of their residency in neonatology. A higher effectiveness would be expected if catheterization were performed by an expert, as evinced in the research of Lloreda-Garcia et al.1 and Froehlich et al.,30 and therefore, the cost-effectiveness ratio could be higher.
Limitations of the study. We conducted the study in a tertiary care teaching hospital in the public health system in Mexico, and therefore our findings may not be extrapolated to hospitals of different characteristics. It would be relevant to corroborate these findings in private practice settings. In addition, while patients were not randomly allocated to each type of procedure due to the observational nature of the study, we were able to verify that potential confounders such as gestational age, birth weight, the Apgar scores and blood gases were equivalent in both groups. On the other hand, since the decision to choose the approach to catheterization was made by the neonatologist treating the patient, it would be fair to assume that only practitioners knowledgeable of the ultrasound-guided procedure would choose it, which may have resulted in selection bias, although, as we just noted, both groups had comparable clinical characteristics. Future research should include performance of prospective studies with random allocation to each type of procedure (ultrasound-guided or conventional) after training all neonatologists that could be involved in catheterization. Another limitation is the restriction in the available data inherent in retrospective studies. However, we only needed to eliminate 2 patients, 1 from each cohort. Lastly, while the effectiveness index applied in this study was based on the methodology of a similar study on cost-effectiveness,21 we are aware of the subjectivity involved in the weighting of the factors established by neonatology experts.
ConclusionsThe use of ultrasonography to guide umbilical vein catheterization is more efficient than conventional catheterization, for while it involves a greater use of economic resources it is more effective. Specifically, it was associated with a lower incidence of complications and a higher correct catheter tip positioning rate. We recommend considering the routine use of ultrasound to guide umbilical catheter insertion in teaching hospitals.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the student Karen Ailyn Salas Ramírez for her help in carrying out this project.
Please cite this article as: Guzmán-de la Garza FJ, Laredo-Flores AD, Cárdenas-del Castillo B, Cordero-Franco HF, Salinas-Martínez AM, Fernández-Garza NE, et al. Cateterización venosa umbilical guiada por ultrasonografía: un análisis de coste-efectividad. An Pediatr (Barc). 2020;92:215–221.