Telemedicine is an attractive option for the follow-up of paediatric patients with SARS-CoV-2 infection. The aim of this article is to describe the experience with telephone consultations in a tertiary hospital.
Patients and methodsRetrospective descriptive study of children with confirmed or probable diagnosis of COVID-19 attended by telephone consultations in Hospital La Paz (Madrid) between March and June 2020. Patients were referred from the Emergency Department after being discharged from the hospital. Telephone consultations were made every 48 h until symptoms resolved, then weekly until completing 14 days without symptoms.
ResultsA total of 72 children were included, with median age of 83.5 months [IQR = 16.3−157.5]. Of those 46 (63.9%) were male, and 14 (19.4%) had comorbidities. There were 32 (44.4%) hospital admissions. COVID-19 diagnosis was confirmed in 33 children by RT-PCR, and in 7 by serology tests. The seroconversion rate was 67.7% in those patients with a positive RT-PCR. Other infections were found in 7 patients (5 Mycoplasma pneumoniae, 1 parvovirus, and 1 CMV). Median symptom duration was 25.5 days [IQR = 13.8−37], while median follow-up duration was 28 days [IQR = 21−39]. The median number of telephone consultations per patient was 6 [IQR = 4−8]. Clinical worsening was reported in 19 (26.4%) during follow-up, and 14 (19.4%) were re-evaluated in the Emergency Department. One patient required hospital admission, but he had a favourable outcome.
ConclusionsChildren with suspected SARS-CoV-2 infection should be followed-up due to prolonged duration of symptoms, and the risk of clinical deterioration. Telephone consultations are a useful and safe alternative for the follow-up of patients with mild symptoms, and for children discharged from the hospital.
El seguimiento telefónico es una posible alternativa para la atención médica de niños con COVID-19. Nuestro objetivo es describir la experiencia del seguimiento telemático realizado en un hospital terciario.
Pacientes y métodosEstudio descriptivo retrospectivo de los niños con diagnóstico confirmado o probable de COVID-19 atendidos en la consulta de seguimiento del Hospital La Paz entre marzo y junio de 2020. Se realizaron llamadas cada 48 horas hasta desaparecer los síntomas y posteriormente semanales hasta estar 14 días asintomáticos.
ResultadosSe incluyeron 72 niños con mediana de edad de 83,5 meses [RIC = 16,3-157,5]. 46 eran varones (63,9%) y 14 tenían comorbilidades (19,4%). 32 pacientes (44,4%) habían requerido ingreso hospitalario. Se confirmó diagnóstico de COVID-19 en 33 niños por PCR y en 7 por serología. De los confirmados por PCR seroconvirtieron el 67,7%. Se demostraron otras etiologías en 7 pacientes (5 Mycoplasma pneumoniae, 1 parvovirus y 1 CMV).
La mediana de duración de síntomas fue 25,5 días [RIC = 13,8-37], con mediana de tiempo de seguimiento de 28 días [RIC = 21-39]. Se realizó una mediana de 6 llamadas [RIC = 4-8] por niño. 19 pacientes (26,4%) refirieron empeoramiento en el seguimiento, precisando 14 (19,4%) ser reevaluados en urgencias. Un niño necesitó reingresar siendo la evolución favorable.
ConclusionesAnte sospecha de COVID-19 es recomendable el seguimiento médico por la prolongada duración de síntomas y el riesgo de empeoramiento. Las consultas telefónicas son útiles y seguras para el seguimiento de casos leves y pacientes hospitalizados tras el alta, permitiendo reducir controles presenciales y el consumo de otros recursos.
Since December 2019, infection by SARS-CoV-2 has spread rapidly throughout the world, giving rise to a pandemic of coronavirus disease (COVID-19).1 After the initial outbreak in China, Europe became the epicentre of the pandemic.2 The available data suggest that the course of COVID-19 in children tends to be mild, although a small percentage may develop severe forms of disease that may even require intensive care.3–5
Spain has been one of the countries most affected by the pandemic, with 285 430 confirmed cases as of July 30, 2020, and the Community of Madrid (CAM) was the region with the highest incidence.6 At the peak of the pandemic, hospital-based paediatric care was restricted to the Hospital Universitario La Paz (HULP) and the Hospital Universitario Infantil Niño Jesús,7 reallocating all other paediatric emergency departments and inpatient wards to the care of adult patients with COVID-19.
Due to the overburdening of the health care system caused by the pandemic, and in an attempt to contain the spread of the virus, multiple clinical guidelines have proposed that the follow-up of patients with mild COVID-19 that do not require hospitalization and the post-discharge follow-up of patients that required hospitalization be conducted by telephone.8–10
The aim of our study was to describe and evaluate the remote follow-up of paediatric patients with diagnosed or suspected COVID-19 and summarise their clinical characteristics and outcomes.
Sample and methodsWe conducted a retrospective descriptive study. We reviewed the health records of patients aged 0–18 years with a confirmed or probable diagnosis of COVID-19 managed in the telephone follow-up clinic of the HULP between March 20 and June 20 of 2020. The study was approved by the Clinical Research Ethics Committee of the HULP (file PI-4212). We sought informed consent for participation in the study by telephone and documented it in the health records. All the data retrieved from electronic health records were anonymised in the database prior to their analysis.
We included all paediatric patients with a confirmed or probable diagnosis of COVID-19 managed in the telephone follow-up clinic whose parents or who themselves, in case they were mature enough, consented to participation. We excluded cases in which telephone follow-up was not possible.
We defined confirmed diagnosis (confirmed COVID-19) as a positive SARS-CoV-2 polymerase chain reaction (PCR) test in a nasal or throat swab sample, or a positive SARS-CoV-2 antibody test.
We defined probable diagnosis (unconfirmed COVID-19) as 2 or more of the following:
- -
Clinical criteria: any of the following,
- a
Acute respiratory infection.
- b
Fever without source or accompanied by severe headache or anosmia/dysgeusia or distal acral chilblain-like lesions.
- c
Clinical presentation compatible with multisystem inflammatory syndrome in children (MIS-C) temporally related to COVID-19 applying the criteria of the World Health Organization (WHO).11
- a
- -
Laboratory criteria: at least 2 findings of blood testing indicative of COVID-19 (D-dimer elevation, lymphopenia, hyperferritinaemia or elevation of interleukin-6).
- -
Radiologic criterion: chest imaging features compatible with COVID-19.
- -
Epidemiological criteria: recent and close contact (>15 min at less than 2 m distance) with a confirmed COVID-19 case or with health care staff with a history of high-risk exposure.
We defined the following syndromes based on the clinical manifestations that dominated the presentation: respiratory syndrome, fever without source, MIS-C, malaise (prolonged low-grade fever, severe asthenia or headache), asymptomatic, other.
The telephone follow-up clinic was set up in the premises of the paediatrics, infectious and tropical diseases outpatient clinics of the HULP. It was created specifically for the follow-up of COVID-19 patients on March 20, 2020. Visits for paediatric patients were scheduled from the emergency department or on hospital discharge. The paediatric emergency department only referred select patients that belonged to risk groups or who required close monitoring, but all paediatric patients that had been hospitalised were scheduled for follow-up after discharge. Telephone follow-up appointments were conducted using a structured questionnaire (Appendix B) that explored symptoms and treatment, and responses were entered in the electronic health records. The phone calls were made by a paediatrician of the Department of Paediatric Infectious Diseases specifically trained for the purpose.
During the follow-up, calls were scheduled every 24–72 hours for as long as the patient had active symptoms or was receiving specific treatment, and subsequently every 5–7 days until the patient had been asymptomatic for 14 consecutive days. Families were provided with a contact number in case they needed to consult or bring forward the following visit. The paediatrician responsible for the follow-up referred patients for additional in-person evaluations in the paediatric emergency department or for diagnostic tests if he considered it necessary.
We offered all patients a SARS-CoV-2 antibody test to confirm the diagnosis or to assess for seroconversion. We used chemiluminescence-based tests by Abbott, Siemens and Vircell for the purpose.
Statistical analysisWe collected data on epidemiological variables, symptoms and their duration, diagnostic tests and treatments used, the duration of follow-up, the number of calls made and clinical outcomes during the follow-up. We assessed worsening in patients based on reports by the family, return visits to the emergency department and the need of readmission. We have expressed qualitative variables as percentages and quantitative variables as median and interquartile range (IQR).
We compared the characteristics of patients with confirmed COVID-19 and patients with a probable diagnosis. We assessed for differences in categorical variables between groups with the Fisher exact test. We assessed for differences in continuous variables with the Mann-Whitney U test. The level of significance was set at 5% (P ≤ .05).
The statistical analysis was performed with the software IBM SPSS Statistics for Windows, version 22.0 (IBM Corp, Armonk, NY, USA).
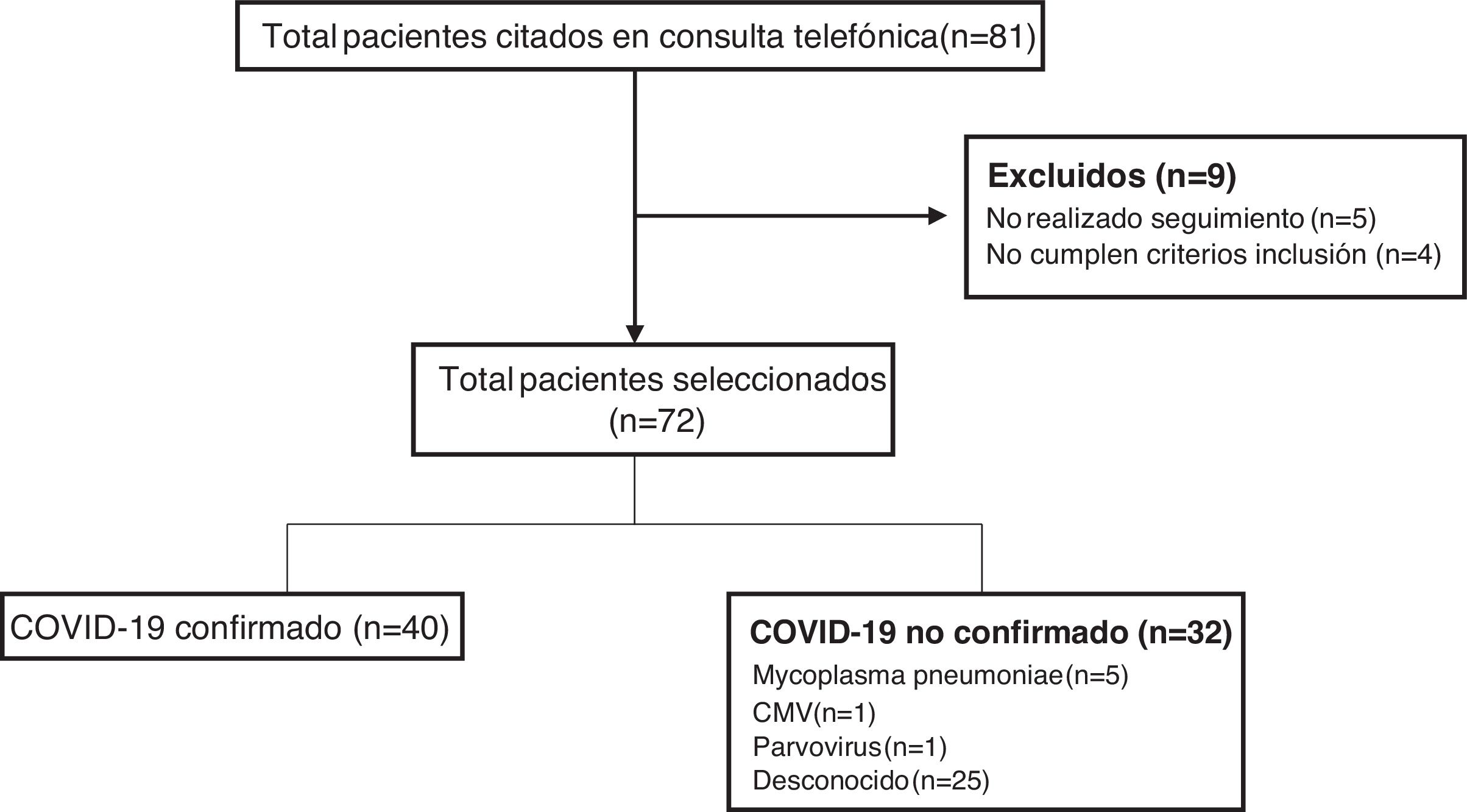
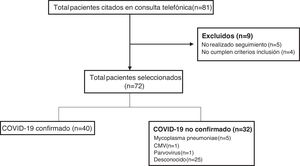
ResultsDuring the period under study, the remote follow-up clinic scheduled appointments with a total of 81 patients, of who 72 met the inclusion criteria, participated in the follow-up and were included in the study (Fig. 1). Forty patients (55.5%) had confirmed COVID-19 (confirmation by SARS-CoV-2 PCR in 33 and antibody test in 7), while a causative agent was not identified in 25 (34.7%). Other pathogens detected in patients were: Mycoplasma pneumoniae in 5 patients (6.9%), cytomegalovirus (CMV) in 1 (1.4%) and parvovirus in 1 (1.4%).
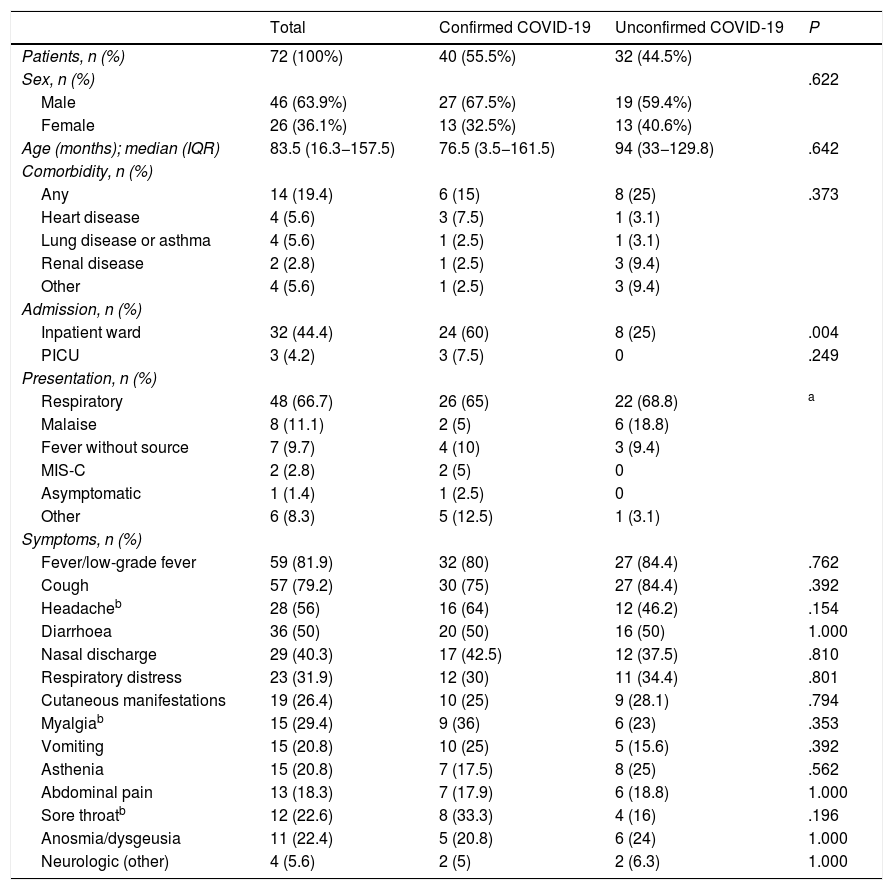
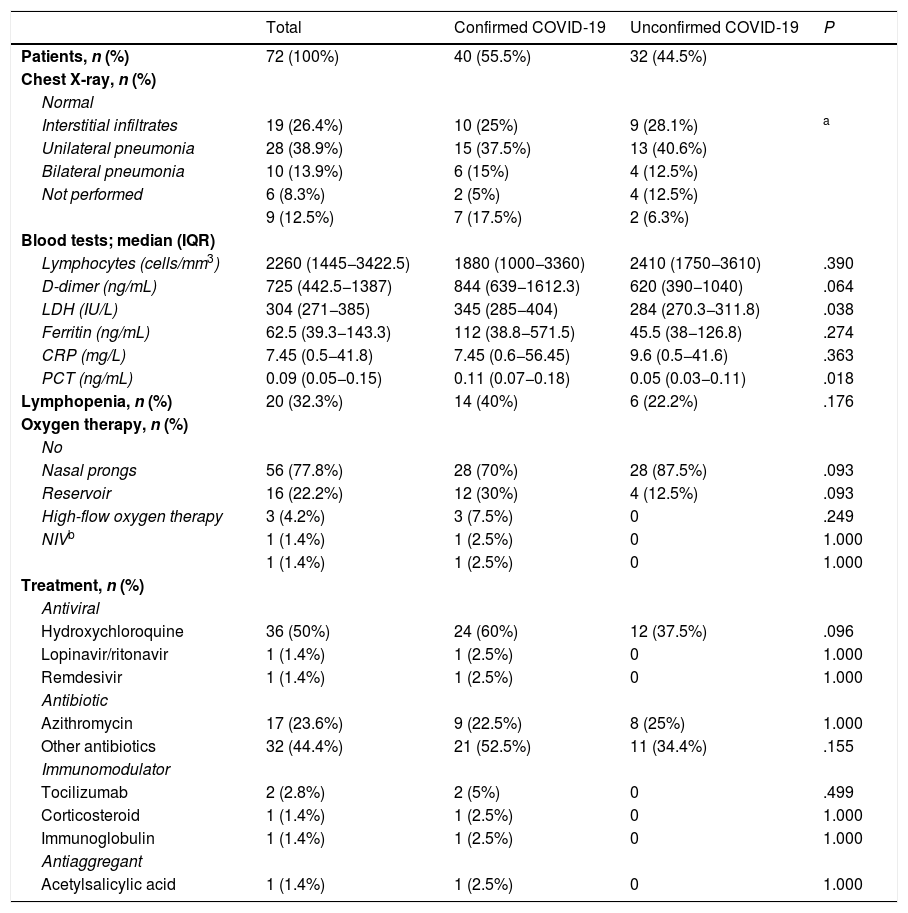
Table 1 summarises the epidemiological characteristics and clinical manifestations of the patients. Table 2 presents the diagnostic tests and treatments used. We found a higher proportion of admissions in patients with confirmed COVID-19 (P ≤ .004). IN addition, patients with confirmed COVID-19 had significantly higher levels of lactate dehydrogenase (P ≤ .038) and procalcitonin (P ≤ .018). We did not find statistically significant differences between groups in the remaining variables.
Epidemiological characteristics and clinical presentation of the patients.
| Total | Confirmed COVID-19 | Unconfirmed COVID-19 | P | |
|---|---|---|---|---|
| Patients, n (%) | 72 (100%) | 40 (55.5%) | 32 (44.5%) | |
| Sex, n (%) | .622 | |||
| Male | 46 (63.9%) | 27 (67.5%) | 19 (59.4%) | |
| Female | 26 (36.1%) | 13 (32.5%) | 13 (40.6%) | |
| Age (months); median (IQR) | 83.5 (16.3−157.5) | 76.5 (3.5−161.5) | 94 (33−129.8) | .642 |
| Comorbidity, n (%) | ||||
| Any | 14 (19.4) | 6 (15) | 8 (25) | .373 |
| Heart disease | 4 (5.6) | 3 (7.5) | 1 (3.1) | |
| Lung disease or asthma | 4 (5.6) | 1 (2.5) | 1 (3.1) | |
| Renal disease | 2 (2.8) | 1 (2.5) | 3 (9.4) | |
| Other | 4 (5.6) | 1 (2.5) | 3 (9.4) | |
| Admission, n (%) | ||||
| Inpatient ward | 32 (44.4) | 24 (60) | 8 (25) | .004 |
| PICU | 3 (4.2) | 3 (7.5) | 0 | .249 |
| Presentation, n (%) | ||||
| Respiratory | 48 (66.7) | 26 (65) | 22 (68.8) | a |
| Malaise | 8 (11.1) | 2 (5) | 6 (18.8) | |
| Fever without source | 7 (9.7) | 4 (10) | 3 (9.4) | |
| MIS-C | 2 (2.8) | 2 (5) | 0 | |
| Asymptomatic | 1 (1.4) | 1 (2.5) | 0 | |
| Other | 6 (8.3) | 5 (12.5) | 1 (3.1) | |
| Symptoms, n (%) | ||||
| Fever/low-grade fever | 59 (81.9) | 32 (80) | 27 (84.4) | .762 |
| Cough | 57 (79.2) | 30 (75) | 27 (84.4) | .392 |
| Headacheb | 28 (56) | 16 (64) | 12 (46.2) | .154 |
| Diarrhoea | 36 (50) | 20 (50) | 16 (50) | 1.000 |
| Nasal discharge | 29 (40.3) | 17 (42.5) | 12 (37.5) | .810 |
| Respiratory distress | 23 (31.9) | 12 (30) | 11 (34.4) | .801 |
| Cutaneous manifestations | 19 (26.4) | 10 (25) | 9 (28.1) | .794 |
| Myalgiab | 15 (29.4) | 9 (36) | 6 (23) | .353 |
| Vomiting | 15 (20.8) | 10 (25) | 5 (15.6) | .392 |
| Asthenia | 15 (20.8) | 7 (17.5) | 8 (25) | .562 |
| Abdominal pain | 13 (18.3) | 7 (17.9) | 6 (18.8) | 1.000 |
| Sore throatb | 12 (22.6) | 8 (33.3) | 4 (16) | .196 |
| Anosmia/dysgeusia | 11 (22.4) | 5 (20.8) | 6 (24) | 1.000 |
| Neurologic (other) | 4 (5.6) | 2 (5) | 2 (6.3) | 1.000 |
Diagnostic tests and treatments used.
| Total | Confirmed COVID-19 | Unconfirmed COVID-19 | P | |
|---|---|---|---|---|
| Patients, n (%) | 72 (100%) | 40 (55.5%) | 32 (44.5%) | |
| Chest X-ray, n (%) | ||||
| Normal | ||||
| Interstitial infiltrates | 19 (26.4%) | 10 (25%) | 9 (28.1%) | a |
| Unilateral pneumonia | 28 (38.9%) | 15 (37.5%) | 13 (40.6%) | |
| Bilateral pneumonia | 10 (13.9%) | 6 (15%) | 4 (12.5%) | |
| Not performed | 6 (8.3%) | 2 (5%) | 4 (12.5%) | |
| 9 (12.5%) | 7 (17.5%) | 2 (6.3%) | ||
| Blood tests; median (IQR) | ||||
| Lymphocytes (cells/mm3) | 2260 (1445−3422.5) | 1880 (1000−3360) | 2410 (1750−3610) | .390 |
| D-dimer (ng/mL) | 725 (442.5−1387) | 844 (639−1612.3) | 620 (390−1040) | .064 |
| LDH (IU/L) | 304 (271−385) | 345 (285−404) | 284 (270.3−311.8) | .038 |
| Ferritin (ng/mL) | 62.5 (39.3−143.3) | 112 (38.8−571.5) | 45.5 (38−126.8) | .274 |
| CRP (mg/L) | 7.45 (0.5−41.8) | 7.45 (0.6−56.45) | 9.6 (0.5−41.6) | .363 |
| PCT (ng/mL) | 0.09 (0.05−0.15) | 0.11 (0.07−0.18) | 0.05 (0.03−0.11) | .018 |
| Lymphopenia, n (%) | 20 (32.3%) | 14 (40%) | 6 (22.2%) | .176 |
| Oxygen therapy, n (%) | ||||
| No | ||||
| Nasal prongs | 56 (77.8%) | 28 (70%) | 28 (87.5%) | .093 |
| Reservoir | 16 (22.2%) | 12 (30%) | 4 (12.5%) | .093 |
| High-flow oxygen therapy | 3 (4.2%) | 3 (7.5%) | 0 | .249 |
| NIVb | 1 (1.4%) | 1 (2.5%) | 0 | 1.000 |
| 1 (1.4%) | 1 (2.5%) | 0 | 1.000 | |
| Treatment, n (%) | ||||
| Antiviral | ||||
| Hydroxychloroquine | 36 (50%) | 24 (60%) | 12 (37.5%) | .096 |
| Lopinavir/ritonavir | 1 (1.4%) | 1 (2.5%) | 0 | 1.000 |
| Remdesivir | 1 (1.4%) | 1 (2.5%) | 0 | 1.000 |
| Antibiotic | ||||
| Azithromycin | 17 (23.6%) | 9 (22.5%) | 8 (25%) | 1.000 |
| Other antibiotics | 32 (44.4%) | 21 (52.5%) | 11 (34.4%) | .155 |
| Immunomodulator | ||||
| Tocilizumab | 2 (2.8%) | 2 (5%) | 0 | .499 |
| Corticosteroid | 1 (1.4%) | 1 (2.5%) | 0 | 1.000 |
| Immunoglobulin | 1 (1.4%) | 1 (2.5%) | 0 | 1.000 |
| Antiaggregant | ||||
| Acetylsalicylic acid | 1 (1.4%) | 1 (2.5%) | 0 | 1.000 |
CRP, C-reactive protein; IQR, interquartile range; LDH, lactate dehydrogenase; NIV, non-invasive mechanical ventilation; PCT, procalcitonin; COVID-19, coronavirus disease caused by SARS-CoV-2.
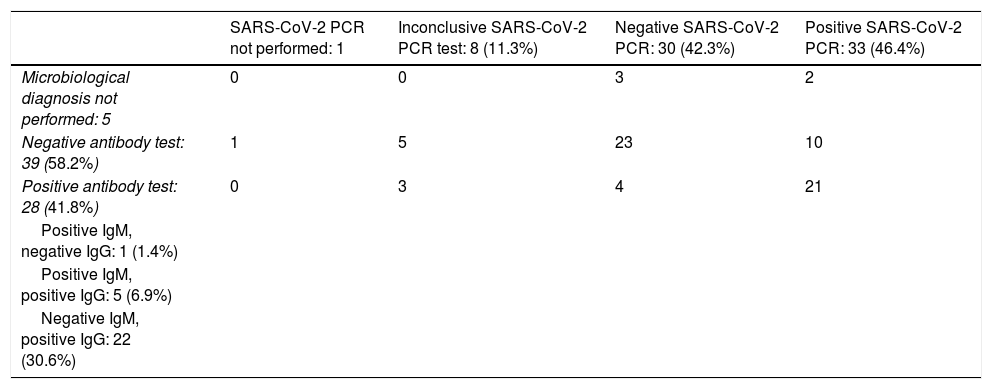
Table 3 presents the results of the tests performed for microbiological diagnosis of COVID-19. The SARS-CoV-2 PCR test was performed in 71 patients (98.6%) and positive in 33 (46.5%). The SARS-CoV-2 antibody test was performed in 67 patients (93.1%), with detection of antibodies in 28 (41.8%). Of the 31 children with a positive SARS-CoV-2 PCR test that underwent antibody testing, 21 (67.7%) were found to have seroconverted. In addition, antibody testing allowed diagnosis of COVID-19 in 4 children with negative PCR tests and in 3 children with inconclusive PCR test results.
Tests for microbiological diagnosis of COVID-19.
| SARS-CoV-2 PCR not performed: 1 | Inconclusive SARS-CoV-2 PCR test: 8 (11.3%) | Negative SARS-CoV-2 PCR: 30 (42.3%) | Positive SARS-CoV-2 PCR: 33 (46.4%) | |
|---|---|---|---|---|
| Microbiological diagnosis not performed: 5 | 0 | 0 | 3 | 2 |
| Negative antibody test: 39 (58.2%) | 1 | 5 | 23 | 10 |
| Positive antibody test: 28 (41.8%) | 0 | 3 | 4 | 21 |
| Positive IgM, negative IgG: 1 (1.4%) | ||||
| Positive IgM, positive IgG: 5 (6.9%) | ||||
| Negative IgM, positive IgG: 22 (30.6%) |
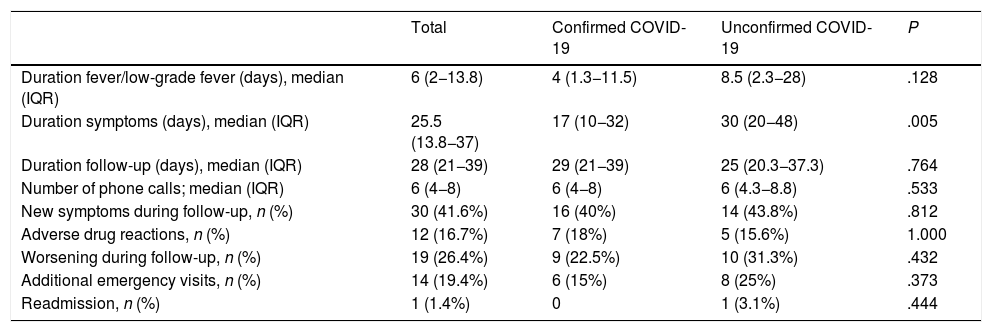
When it came to the follow-up by telephone (Table 4), we observed a significantly longer total duration of symptoms in patients with unconfirmed COVID-19 (P ≤ .005). Female sex was associated with a higher frequency of worsening (P ≤ .028) and emergency visits (P ≤ .027) during the follow-up. Thirty patients (41.7%) reported development of new symptoms during the follow-up: gastrointestinal symptoms in 14 (diarrhoea or abdominal pain), respiratory symptoms in 6, cutaneous manifestations in 4 (exanthema, desquamation or chilblain-like lesions in toes or fingers), recurrence of fever in 3 and other symptoms in 7. The gastrointestinal symptoms of 12 of the 14 patients with these manifestations (85.7%) could probably be attributed to treatment with amoxicillin/clavulanic acid or hydroxychloroquine. Fourteen patients (19.4%) required re-evaluation at the paediatric emergency department during the follow-up. A boy aged 3 years that was followed up by the Department of Rheumatology due to a limp and recurrent fever required readmission 1 month after discharge from the telephone follow-up clinic due to manifestations compatible with MIS-C. The diagnosis of COVID-19 was not confirmed, and the patient had a favourable outcome without requiring specific treatment for the disease. None of the other patients required readmission.
Telephone follow-up.
| Total | Confirmed COVID-19 | Unconfirmed COVID-19 | P | |
|---|---|---|---|---|
| Duration fever/low-grade fever (days), median (IQR) | 6 (2−13.8) | 4 (1.3−11.5) | 8.5 (2.3−28) | .128 |
| Duration symptoms (days), median (IQR) | 25.5 (13.8−37) | 17 (10−32) | 30 (20−48) | .005 |
| Duration follow-up (days), median (IQR) | 28 (21−39) | 29 (21−39) | 25 (20.3−37.3) | .764 |
| Number of phone calls; median (IQR) | 6 (4−8) | 6 (4−8) | 6 (4.3−8.8) | .533 |
| New symptoms during follow-up, n (%) | 30 (41.6%) | 16 (40%) | 14 (43.8%) | .812 |
| Adverse drug reactions, n (%) | 12 (16.7%) | 7 (18%) | 5 (15.6%) | 1.000 |
| Worsening during follow-up, n (%) | 19 (26.4%) | 9 (22.5%) | 10 (31.3%) | .432 |
| Additional emergency visits, n (%) | 14 (19.4%) | 6 (15%) | 8 (25%) | .373 |
| Readmission, n (%) | 1 (1.4%) | 0 | 1 (3.1%) | .444 |
Eight patients (11.1%) presented with malaise. Two received a COVID-19 diagnosis and 1 a diagnosis of parvovirus infection, whereas the aetiology remained unknown in all other cases. Patients that presented with malaise were characterised by a significantly longer duration of symptoms (P ≤ .005), with a median duration of low-grade fever of 53.5 days (IQR, 12.3–64.5) and of symptoms overall of 60 days (IQR, 37–70). These patients were preadolescent or adolescent, with a median age of 142 months (IQR, 117.8–166.8), with a uniform sex distribution (50% male). When we compared this group with the rest of the patients, we found fewer abnormal results or features in laboratory and imaging tests, and none required admission. Three of these patients (37.5%) received some form of pharmacotherapy (hydroxychloroquine, azithromycin or a beta-lactam antibiotic). They also required longer follow-up (P ≤ .018), with a median duration of 52.5 days (IQR, 25–60.5) and a median of 11 calls (IQR, 6.3–19) per patient. Four (50%) reported worsening during follow-up and visited the emergency department. All had favourable outcomes.
When it came to treatment, hydroxychloroquine was associated with a shorter duration of fever (P ≤ .023), decreased frequency of worsening during follow-up (P ≤ .016) and fewer visits to the paediatric emergency department (P ≤ .017). In contrast, we observed a longer duration of symptoms in patients treated with azithromycin (P ≤ .037).
DiscussionTo date, there is little evidence on the remote follow-up of paediatric patients with suspected COVID-19. Our study describes satisfactory and safe follow-up by telephone based off a tertiary care hospital in 72 paediatric patients. The salient findings were the long duration of symptoms, with a median of 25.5 days, and the worsening reported by 19 of the patients (26.4%), of who 14 (19.4%) required a new in-person assessment. These circumstances explain the long duration of follow-up and the performance of a median of 6 follow-up phone calls per patient. Notwithstanding, readmission was very infrequent and associated with the presence of comorbidities, and all patients had favourable outcomes.
In interpreting the data of this study, it is important to take into account that the patients were referred from the paediatric emergency department, which only referred patients at greater risk of having unfavourable outcomes, or at discharge from hospital. Thus, patients in our study probably had more symptoms and more severe forms of disease compared to patients in studies including the full spectrum of patients managed in paediatric emergency departments, primary care or identified through contact tracing. This would explain the higher prevalence of symptoms we observed compared to previous reports in the literature.3–5,12
In agreement with previous paediatric case series,3–5 the prevailing presentation of children infected by SARS-CoV-2 was respiratory, and fever and cough were the most frequent symptoms. Headache and diarrhoea were also common symptoms, although in some cases the latter was associated with pharmacological treatment. When we compared patients with confirmed COVID-19 and those without confirmation, we did not find differences in the frequency of symptoms described in the literature as characteristic of COVID-19, such as anosmia and certain cutaneous manifestations (desquamation or chilblain-like lesions in hands and feet).13–16
The subset of patients that we defined as presenting with malaise stood out on account of the long duration of symptoms. These patients required significantly longer follow-up, were more likely to visit the emergency department for reassessment and need a more extensive workup (including testing for Epstein-Barr virus, CMV, toxoplasma, etc.), in spite of which an aetiological diagnosis was only achieved in 3 of them. We ought to highlight the significant impairment resulting from the severe headache and weakness experienced by some of these patients, while others only experienced persistent low-grade fever that they tolerated well, in the absence of any other symptoms. It is possible that some of these manifestations, like headache or asthenia, were exacerbated by the stress and anxiety caused by the pandemic and prolonged confinement.17 There have been studies in adults18,19 that also described persistent symptoms in patients with COVID-19, most frequently asthenia, headache and respiratory complaints.
In our cohort, we found a higher proportion of hospital admissions in the group of children with confirmed COVID-19. This could be explained by the higher nasopharyngeal viral load found in patients with severe disease,20 which would increase the diagnostic yield of the PCR test. Another factor that could have been at play is that early in the pandemic, when the disease was not known, there was a lower threshold for admission of patients with a COVID-19 diagnosis. In contrast, we found a significantly longer duration of symptoms in patients with an unconfirmed diagnosis of COVID-19. This could be due to a higher level of anxiety stemming from the uncertainty regarding the disease.
Although the suspicion was strong in all the patients, the SARS-CoV-2 PCR test was only positive in 33 cases (46.5%). The SARS-CoV-2 PCR test is the most frequently used test for diagnosis during the acute phase of COVID-19. It offers a high specificity, but its sensitivity varies based on different factors (type of specimen, time elapsed from onset, viral load, technique used to collect, transport and process the sample) and it may give rise to false negatives.21–23 Thus, there may have been missed cases of COVID-19 that we were unable to diagnose in the cohort.
Since the viral load peaks at the onset of symptoms or a few days later,20 antibody testing may be useful for diagnosis of patients that did not undergo a SARS-CoV-2 PCR test in the early stage of the infection.24,25 In our cohort, SARS-CoV-2 antibody testing achieved diagnosis of 7 patients (18.4%) with negative of inconclusive PCR results. However, seroconversion was only detected in 21 (67.7%) of the 31 children with a positive PCR that underwent an antibody test. This frequency was substantially lower than the frequency reported in studies conducted in adults,26,27 in which serological testing detected antibodies in nearly all cases of infection. Thus, it would be reasonable to deduce that SARS-CoV-2 antibody testing is less useful in paediatric patients compared to adults for diagnosis of COVID-19. In any case, the absence of antibodies does not necessarily imply absence of an adaptive immune response, as recent studies have found evidence of specific cell-mediated immune responses against SARS-CoV-2 in patients with negative antibody tests.28,29
Hydroxychloroquine was the drug used most frequently in the cohort. Although assessing its efficacy was not the aim of our study, we found a shorter duration of fever and a lower proportion of worsening during the follow-up in patients that received this treatment. The most recent evidence30–32 seems to suggest that this drug is not beneficial for treatment of COVID-19, so these findings may have been due to the placebo effect of treatment. On the other hand, we found that treatment with azithromycin was significantly associated with a longer duration of symptoms. This is probably due to the common use of this antimicrobial agent for rescue treatment of patients with prolonged respiratory symptoms, rather than the drug itself worsening disease outcomes. In other words, azithromycin is probably prescribed in patients whose symptoms last longer.
The main limitation of our study is that the aetiological agent could not be identified in 25 (34.7%) of our patients. As we noted above, this is probably due to the limitations of the diagnostic tests currently available for microbiological diagnosis of COVID-19. There may have been other cases of COVID-19 that we were unable to diagnose. Furthermore, a full aetiological investigation could not be performed in many patients during the peak of the pandemic because the health care system was overwhelmed, so we may have missed infections or coinfections by other pathogens.
Our sample was not representative of the full spectrum of disease caused by SARS-CoV-2 in the paediatric population, as we did not include patients with milder disease managed at the primary care level. Similarly, there were also patients with comorbidities managed by the corresponding specialists that were consequently not included in the cohort under study.
Another aspect to consider is the intrinsic nature of data collection through the telephone, as the subjective perception of interviewed family members may have been a source of bias in our interpretation of symptoms and their severity. At the same time, the absence of a control group prevented the possibility of determining whether telephone follow-up improved patient outcomes or decreased the frequency of emergency visits.
ConclusionsFollow-up of children with suspected COVID-19 is advisable, given that symptoms tend to be prolonged and that up to 26.4% experience worsening of the course of disease. Remote visits are a useful and safe strategy that can reduce the frequency of in-person visits and use of additional resources. It is a valid alternative for management of mild cases of COVID-19 and for post-discharge follow-up of patients admitted to hospital.
Antibody tests can be useful for diagnosis in paediatric patients with presentations compatible with COVID-19 and negative SARS-CoV-2 PCR test results, although they offer a lower yield compared to adults due to the lower rate of seroconversion in the paediatric population.
Conflicts of interestThe authors have no conflicts of interest to declare.
Members of the Working Group on SARS-CoV-2 of the Department of Paediatrics of the Hospital Universitario La Paz
Laura García Espinosa, Clara Buitrago Gil, María de Ceano-Vivas La Calle, Teresa del Rosal Rabes, Ana Mendez-Echevarría, Fernando Baquero Artigao, Talía Sainz Costa, María José Mellado Peña.
Please cite this article as: Nogueira López J, Grasa Lozano C, Ots Ruiz C, Alonso García L, Falces-Romero I, Calvo C, et al. Seguimiento telemático de COVID-19: experiencia de un hospital terciario. An Pediatr (Barc). 2021;95:336–344.
The members of the SARS-CoV-2 Working Group of the Pediatric Service of Hospital Universitario La Paz are presented in Appendix A.
Previous presentation: the study was accepted for presentation as a poster at the I COVID-19 National Congress, September 13–19, held online.