Newborn screening programs are key players in a country’s public health strategies, preventing the burden of care associated with the screened disorders. Its importance has dramatically intensified in recent years due to the increasing number of disorders that fulfil criteria for screening. Since the 1960s, many countries implemented newborn screening programs that are now, at least in developed countries, universal, well established, and with excellent results. Nevertheless, much work is still to be done, mainly in developing countries of Africa, Asia, and South America. In some European countries, including Spain, uniformity of screening panels between different regions is still a challenge, being a source of health inequalities between citizens. The authors will present the current status of newborn screening programs in Spain and integrate it into the current European and world scenario.
Los programas de cribado neonatal (PCN) son clave en las estrategias de salud pública de una región determinada, establecidas para prevenir los daños asociados a las patologías cribadas. Su importancia se ha intensificado sustancialmente en los últimos años debido al creciente número de trastornos en los que diferentes organismos de evaluación han demostrado el beneficio de su detección temprana para el recién nacido. Desde los años 60-70 del siglo pasado, muchas regiones implementaron de PNC que hoy en día, al menos en los países desarrollados, son universales, bien establecidos y con excelentes resultados. Sin embargo, aún queda mucho por hacer, principalmente en países en vías de desarrollo de África, Asia y América del Sur. En algunos países europeos, incluida España, una mayor uniformidad entre los paneles de cribado de las diferentes regiones continúa siendo un reto, pues conduce a desigualdades en materia de salud. Los autores presentan el estado actual de los PCN en España y lo contextualizan en el escenario real europeo y mundial.
Newborn screening (NBS) programmes are public health interventions for the detection of specific severe congenital diseases in newborns with the aim of treating them before the onset of symptoms. The origins of newborn screening date back to the mid-20th century with the work of Guthrie and Susi,1 who developed a simple, inexpensive and effective test to determine whether newborns had phenylketonuria (PKU).
In order to define the requirements that need to be met to include additional diseases in screening programmes, the World Health Organization published the Wilson and Jungner criteria for screening within Principles and practice of screening for disease in 1968.2 These criteria, defined according to current circumstances, aimed to guarantee that these programmes fulfil their primary objective: maximum benefit with minimum cost.
As time passed, new laboratory tests were developed for detection of additional diseases, among which we ought to emphasise congenital hypothyroidism (CHT). Phenylketonuria and congenital hypothyroidism are the diseases most commonly screened for, and there is substantial variation in the remaining diseases that are included in screening panels. After a few years in which NBS programmes advanced slowly, as additional diseases were included at the pace that technological advances occurred, there was a revolution in these programmes in the early 1990s with the introduction in screening laboratories of tandem mass spectrometry (MS/MS).3 This technique is mainly aimed at the detection of amino acid, organic acid and mitochondrial fatty acid β-oxidation disorders. It is a multi-analyte method that allows the detection and simultaneous measurement of more than 50 metabolites, and thus screening for more than 40 inborn errors of metabolism using a single dry blood sample. The emergence of this technology constituted a paradigm shift, moving from the conventional “one test > one metabolite > one disease” to the MS/MS “one test > multiple metabolites > multiple diseases”. Tandem mass spectrometry has become a key method for detection of inherited metabolic disorders.
This new approach calls for reconsidering the classic criteria established by Wilson and Jungner that are still in use, as they were defined in a different context and therefore need to be adapted. Potentially treatable diseases associated with a high morbidity and mortality could be candidates for inclusion in an expanded newborn screening panel even if their prevalence is low. On the other hand, while the marginal cost of expanding a NBS programme could be relatively small, the use of MS/MS does not necessarily imply the inclusion of every potentially detectable disease. Therefore, while challenging, the cost assessment must be performed taking into account the potential benefits of including rare but treatable diseases that we could now screen for with no direct added costs. Difficulties in the application of the previously defined criteria and differences in their interpretation have arisen with this new status quo and are reflected in the differences that can be found in the diseases included in screening programmes that have already introduced the use of MS/MS. Nevertheless, we ought to highlight that despite the current heterogeneity in screening programmes, the introduction of MS/MS in screening laboratories in and of itself is not debatable, as the use of this technique is already justified by the optimization of PKU testing and the screening for medium-chain acyl-CoA dehydrogenase deficiency (MCADD).4
Differences in the recommendations for screening of metabolic disorders are intrinsically dependent on political, cultural, sociocultural and above all economic factors. The different circumstances of each country or region not only lead to differences in the recommendations regarding the diseases that ought to be screened for, but also in the organization and funding of NBS programmes.5,6
Current situationAll NBS programmes, either in developed countries or in the developing countries that have them, include screening for PKU and CHT or at least one of them. Furthermore, in recent years the coverage of newborn screening by the heel prick test continues to increase, while NBS programmes are including additional diseases that can cause early death, such as severe infection or severe anaemias. When it comes to inherited metabolic disorders, the introduction of MS/MS in NBS programmes allows their differentiation into 2 categories: those that can be detected by MS/MS and those whose detection requires other techniques. The main diseases in the latter group are classic galactosaemia and biotinidase deficiency.
Newborn screening of inborn errors of metabolism by means of MS/MS is widespread in developed countries, and the initial challenges involved in the validation of laboratory techniques and clinical interpretation as well as the clinical follow-up have been resolved satisfactorily.7,8 At the same time, the benefits of screening for certain diseases continues under debate. Within the broad umbrella of inborn errors of metabolism that can be detected by MS/MS, there are some whose screening offers clear and direct benefits to the newborn, and others in which the benefits of screening are not that obvious. One example is the screening of diseases that are not treatable, whose primary objectives could be diagnosis for the purpose of obtaining important information for future genetic counselling of the family or prenatal diagnosis. All of these issues have led to significant discrepancies in the criteria used to establish the diseases to be included in screening.
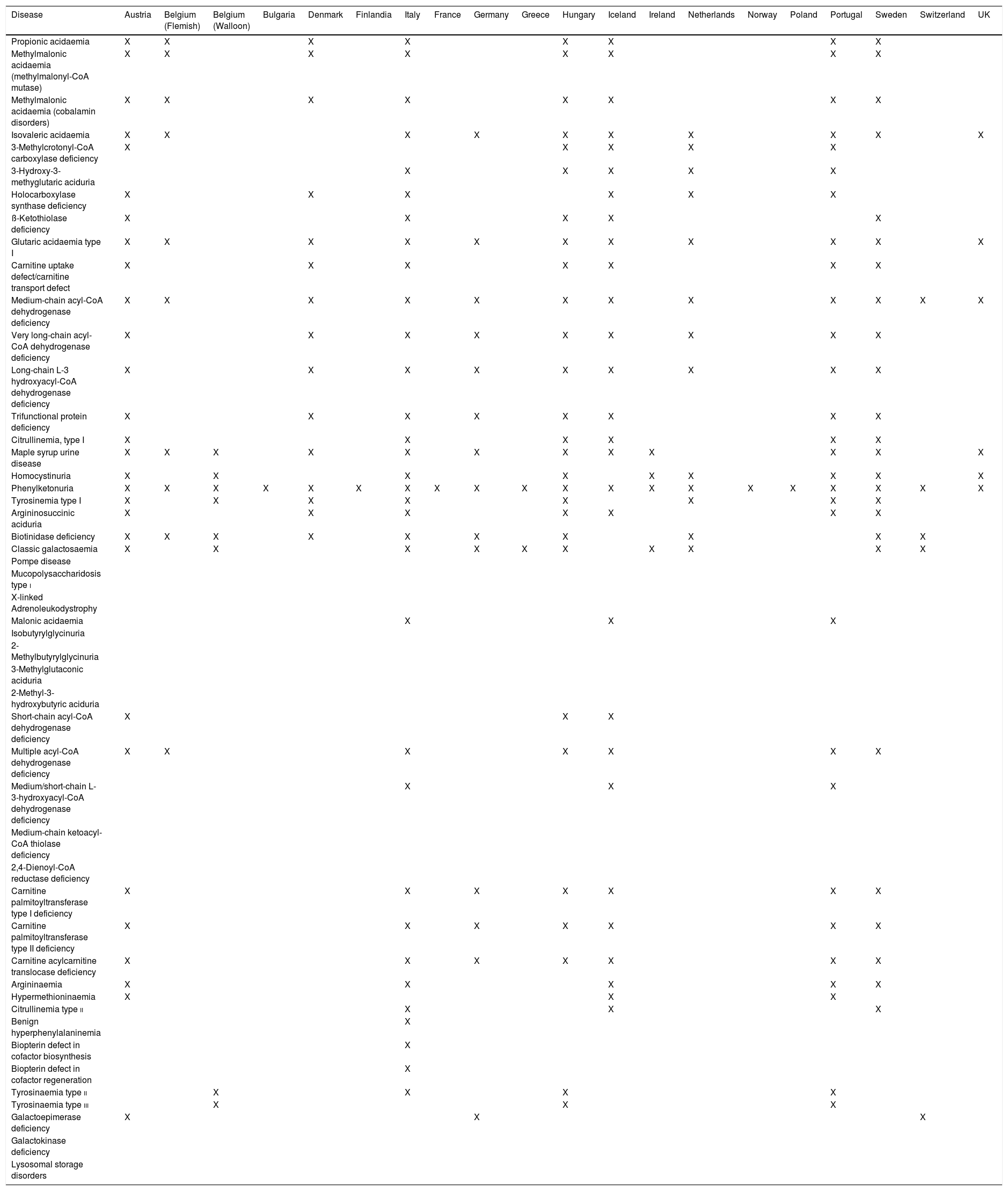
AmericaIn the United States (US), the American College of Medical Genetics published the document newborn screening: toward a uniform screening panel and system in 2006 with the aim of establishing a uniform screening programme across its states.9 The document was developed by a group of experts, who identified 29 diseases as primary targets for screening, of which 20 can be detected by MS/MS. They also identified a second group of 25 diseases considered secondary targets, since the benefits of their detection were less clear, of which 22 were detectable by MS/MS. This was the first study of the kind and it led to the establishment by the United States secretary of health and human services of the recommended uniform screening panel, an established and homogeneous screening programme that includes a large group of diseases. This initial work has served as a reference and is periodically updated with ongoing evaluation of additional diseases that could be included. At the time of this writing, the recommended uniform screening panel includes 35 primary targets and more than 26 secondary targets (Table 1).10 As a result of these recommendations, there is significant homogeneity in the diseases screened in each of the states in the country. Due to this approach to screening, the US is considered one of the most liberal countries when it comes to the interpretation of the Wilson and Jungner criteria. The recommended uniform screening panel is also considered a reference for the purpose of debate and evaluation in other countries.
Diseases for which screening is recommended in the recommended uniform screening panel and by the EU network of experts on newborn screening.
| Disease | Recommended uniform screening panel | [0,4–5]EU network of experts on newborn screening | ||
|---|---|---|---|---|
| Primary target | Secondary target | Disorders that should be screened | Disorders to consider for screening expansion | |
| Propionic acidaemia | X | |||
| Methylmalonic acidaemia (methylmalonyl-CoA mutase) | X | |||
| Methylmalonic acidaemia (cobalamin disorders) | X | |||
| Isovaleric acidaemia | X | X | ||
| 3-Methylcrotonyl-CoA carboxylase deficiency | X | X | ||
| 3-Hydroxy-3-methyglutaric aciduria | X | X | ||
| Holocarboxylase synthase deficiency | X | X | ||
| ß-ketothiolase deficiency | X | |||
| Glutaric acidaemia type I | X | X | ||
| Carnitine uptake defect/carnitine transport defect | X | |||
| Medium-chain acyl-CoA dehydrogenase deficiency | X | X | ||
| Very long-chain acyl-CoA dehydrogenase deficiency | X | X | ||
| Long-chain L-3 hydroxyacyl-CoA dehydrogenase deficiency | X | X | ||
| Trifunctional protein deficiency | X | |||
| Argininosuccinic aciduria | X | |||
| Citrullinemia, type I | X | |||
| Maple syrup urine disease | X | X | ||
| Homocystinuria | X | X | ||
| Phenylketonuria | X | X | ||
| Tyrosinemia type i | X | X | ||
| Biotinidase deficiency | X | |||
| Classic galactosaemia | X | X | ||
| Pompe disease | X | |||
| Mucopolysaccharidosis type i | X | |||
| X-linked adrenoleukodystrophy | X | |||
| Spinal muscular atrophy | X | |||
| Methylmalonic acidaemia with homocystinuria | X | X | ||
| Malonic acidaemia | X | |||
| Isobutyrylglycinuria | X | |||
| 2-Methylbutyrylglycinuria | X | |||
| 3-Methylglutaconic aciduria | X | |||
| 2-Methyl-3-hydroxybutyric aciduria | X | |||
| Short-chain acyl-CoA dehydrogenase deficiency | X | |||
| Multiple acyl-CoA dehydrogenase deficiency | X | X | ||
| Medium/short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency | X | |||
| Medium-chain ketoacyl-CoA thiolase deficiency | X | |||
| 2,4-Dienoyl-CoA reductase deficiency | X | |||
| Carnitine palmitoyltransferase type I deficiency | X | |||
| Carnitine palmitoyltransferase type II deficiency | X | X | ||
| Carnitine acylcarnitine translocase deficiency | X | X | ||
| Argininaemia | X | |||
| Hypermethioninaemia | X | |||
| Citrullinemia type ii | X | |||
| Benign hyperphenylalaninemia | X | |||
| Biopterin defect in cofactor biosynthesis | X | |||
| Biopterin defect in cofactor regeneration | X | |||
| Tyrosinaemia type ii | X | X | ||
| Tyrosinaemia type iii | X | |||
| Galactoepimerase deficiency | X | |||
| Galactokinase deficiency | X | |||
| Lysosomal storage disorders | X | |||
In Canada, newborn screening also includes a considerable number of metabolic disorders, although fewer compared to the US.11 Some countries in Central and South America have high-quality, well-established NBS programmes, especially Costa Rica12 and Uruguay,13 where all newborns are screened for a large number of metabolic disorders by means of MS/MS. However, most screening programmes in South America include a limited number of diseases in addition to PKU, and few regions use MS/MS for newborn screening.
Asia and OceaniaIn these regions, too, the level of development of the economy and the public health systems is reflected in their NBS programmes. For instance, in Australia14 and Japan15 all newborns are screened for a substantial number of metabolic diseases with MS/MS, but many other countries with fewer resources have not instituted any NBS programmes. In China, screening already covers 80% of newborns and includes PKU, and in some regions includes testing by MS/MS.15 In the Middle East, there are countries, such as Qatar or Saudi Arabia, with programmes that screen all newborns for a broad range of metabolic disorders; others that only include 2 diseases in their NBS programme, such as United Arab Emirates and Kuwait, and a third group continues to not have any form of screening programme.16
AfricaWe ought to differentiate between countries in Northern Africa and countries in Sub-Saharan Africa. Egypt has an established NBS programme, with use of MS/MS in part of the population, and other North African countries are developing projects aimed at the establishment of routine newborn screening.16 The situation in Sub-Saharan Africa is quite different, with very few reports of NBS programmes, even in South Africa.17
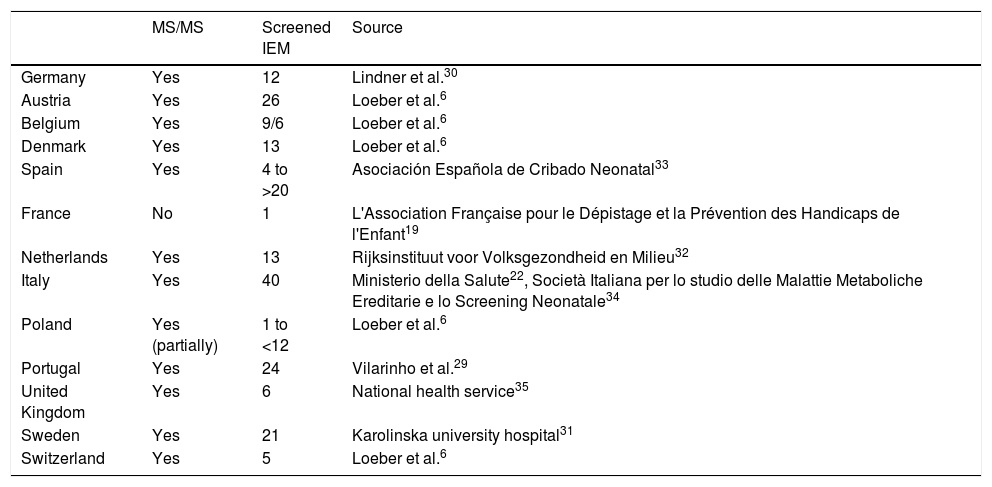
EuropaGeneral situationThe progress of neonatal screening in Europe in the past 50 years has led to screening for PKU in all countries in Western Europe with the addition of screening for biotinidase deficiency and classic galactosaemia in some of them (Table 2). Screening with MS/MS has been gradually introduced in several countries,6 although with significant variation in the diseases included in the screen not only between countries, but also between regions within single countries (Table 3).
Inborn errors of metabolism screened for in different European countries.
| Disease | Austria | Belgium (Flemish) | Belgium (Walloon) | Bulgaria | Denmark | Finlandia | Italy | France | Germany | Greece | Hungary | Iceland | Ireland | Netherlands | Norway | Poland | Portugal | Sweden | Switzerland | UK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Propionic acidaemia | X | X | X | X | X | X | X | X | ||||||||||||
| Methylmalonic acidaemia (methylmalonyl-CoA mutase) | X | X | X | X | X | X | X | X | ||||||||||||
| Methylmalonic acidaemia (cobalamin disorders) | X | X | X | X | X | X | X | X | ||||||||||||
| Isovaleric acidaemia | X | X | X | X | X | X | X | X | X | X | ||||||||||
| 3-Methylcrotonyl-CoA carboxylase deficiency | X | X | X | X | X | |||||||||||||||
| 3-Hydroxy-3-methyglutaric aciduria | X | X | X | X | X | |||||||||||||||
| Holocarboxylase synthase deficiency | X | X | X | X | X | X | ||||||||||||||
| ß-Ketothiolase deficiency | X | X | X | X | X | |||||||||||||||
| Glutaric acidaemia type I | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Carnitine uptake defect/carnitine transport defect | X | X | X | X | X | X | X | |||||||||||||
| Medium-chain acyl-CoA dehydrogenase deficiency | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Very long-chain acyl-CoA dehydrogenase deficiency | X | X | X | X | X | X | X | X | X | |||||||||||
| Long-chain L-3 hydroxyacyl-CoA dehydrogenase deficiency | X | X | X | X | X | X | X | X | X | |||||||||||
| Trifunctional protein deficiency | X | X | X | X | X | X | X | X | ||||||||||||
| Citrullinemia, type I | X | X | X | X | X | X | ||||||||||||||
| Maple syrup urine disease | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Homocystinuria | X | X | X | X | X | X | X | X | X | |||||||||||
| Phenylketonuria | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Tyrosinemia type I | X | X | X | X | X | X | X | X | ||||||||||||
| Argininosuccinic aciduria | X | X | X | X | X | X | X | |||||||||||||
| Biotinidase deficiency | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Classic galactosaemia | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Pompe disease | ||||||||||||||||||||
| Mucopolysaccharidosis type i | ||||||||||||||||||||
| X-linked Adrenoleukodystrophy | ||||||||||||||||||||
| Malonic acidaemia | X | X | X | |||||||||||||||||
| Isobutyrylglycinuria | ||||||||||||||||||||
| 2-Methylbutyrylglycinuria | ||||||||||||||||||||
| 3-Methylglutaconic aciduria | ||||||||||||||||||||
| 2-Methyl-3-hydroxybutyric aciduria | ||||||||||||||||||||
| Short-chain acyl-CoA dehydrogenase deficiency | X | X | X | |||||||||||||||||
| Multiple acyl-CoA dehydrogenase deficiency | X | X | X | X | X | X | X | |||||||||||||
| Medium/short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency | X | X | X | |||||||||||||||||
| Medium-chain ketoacyl-CoA thiolase deficiency | ||||||||||||||||||||
| 2,4-Dienoyl-CoA reductase deficiency | ||||||||||||||||||||
| Carnitine palmitoyltransferase type I deficiency | X | X | X | X | X | X | X | |||||||||||||
| Carnitine palmitoyltransferase type II deficiency | X | X | X | X | X | X | X | |||||||||||||
| Carnitine acylcarnitine translocase deficiency | X | X | X | X | X | X | X | |||||||||||||
| Argininaemia | X | X | X | X | X | |||||||||||||||
| Hypermethioninaemia | X | X | X | |||||||||||||||||
| Citrullinemia type ii | X | X | X | |||||||||||||||||
| Benign hyperphenylalaninemia | X | |||||||||||||||||||
| Biopterin defect in cofactor biosynthesis | X | |||||||||||||||||||
| Biopterin defect in cofactor regeneration | X | |||||||||||||||||||
| Tyrosinaemia type ii | X | X | X | X | ||||||||||||||||
| Tyrosinaemia type iii | X | X | X | |||||||||||||||||
| Galactoepimerase deficiency | X | X | X | |||||||||||||||||
| Galactokinase deficiency | ||||||||||||||||||||
| Lysosomal storage disorders |
Situation of the implementation of screening by means of tandem mass spectrometry in the screening programmes of different European countries, and number of inborn errors of metabolism included in the screening.
| MS/MS | Screened IEM | Source | |
|---|---|---|---|
| Germany | Yes | 12 | Lindner et al.30 |
| Austria | Yes | 26 | Loeber et al.6 |
| Belgium | Yes | 9/6 | Loeber et al.6 |
| Denmark | Yes | 13 | Loeber et al.6 |
| Spain | Yes | 4 to >20 | Asociación Española de Cribado Neonatal33 |
| France | No | 1 | L'Association Française pour le Dépistage et la Prévention des Handicaps de l'Enfant19 |
| Netherlands | Yes | 13 | Rijksinstituut voor Volksgezondheid en Milieu32 |
| Italy | Yes | 40 | Ministerio della Salute22, Società Italiana per lo studio delle Malattie Metaboliche Ereditarie e lo Screening Neonatale34 |
| Poland | Yes (partially) | 1 to <12 | Loeber et al.6 |
| Portugal | Yes | 24 | Vilarinho et al.29 |
| United Kingdom | Yes | 6 | National health service35 |
| Sweden | Yes | 21 | Karolinska university hospital31 |
| Switzerland | Yes | 5 | Loeber et al.6 |
IEM, inborn errors of metabolism; MS/MS, tandem mass spectrometry.
In this context, efforts were made with the aim to harmonise NBS programmes, and the European Commission funded a project, the Evaluation of population newborn screening practices for rare disorders in Member States of the European Union, to analyse newborn screening policies and practices in country, thus setting the foundations to develop guidelines on the subject.6,18 The conclusions of this project are not binding. The evidence that has emerged supporting expanded screening led most countries in Western Europe to include a sizeable group of inherited metabolic disorders in their NBS programmes. There are still differences between different countries, and we ought to highlight the situation in France, where officially there is only routine screening of PKU19 (although the health authorities have recommended the future inclusion of MCADD20).
The situation is quite different in Southeast Europe, as screening is not done to detect any metabolic disorders in Kosovo, Macedonia, Albania, Moldavia and Montenegro, while in Bosnia (different regions), Bulgaria, Croatia, Romania, Serbia and Slovenia screening hardly covers PKU.21
The interregional heterogeneity within some countries is just as large, especially in Spain and in Belgium between the Flemish and Walloon regions. In Italy, a law passed in 2016 mandating the homogenisation and expansion of the metabolic disorders included in the routine NBS programme to close to 40 conditions.22
SpainIn Spain, the first NBS programme was introduced in Granada in 1968 by initiative of professors Federico Mayor-Zaragoza, Magdalena Ugarte and Antonio Martínez Valverde. In 1978, the National Plan for Mental Retardation was established within the Real Patronato de Educación y Atención a Deficientes (Royal Council of Education and Care for Individuals with Disabilities), and several laboratories were established within its framework. Between 1982 and 1983, the authorities of each autonomous region (AR) in Spain took over the management of government-run programmes for the early detection of congenital and metabolic disorders.23
Between 2000 and 2015, there were significant differences in the NBS programmes of the different ARs, as many only included 2 or 3 diseases while others included more than 20.24,25 Each AR independently determined the number of diseases to be screened, and since there was no institution coordinating the development of these regional programmes, significant variation ensued.
With the aim of establishing the actual benefits of the early diagnosis of diseases susceptible of screening, the Federación Española de Fenilcetonuria y otros Trastornos del Metabolismo (Spanish Federation of Phenylketonuria and other Metabolic Disorders), along with a group of health professionals, agreed to review the existing NBS programmes in Spain to develop the broadest-possible consensus on aspects such as the criteria applied to select diseases for inclusion, the establishment of units for the diagnosis, treatment and followup of the detected diseases, and the institution of a national register of affected patients. They developed the consensus document Programas de cribado neonatal en España: Actualización y propuestas de futuro. Documento de consenso (2010) [newborn screening programmes: update and proposals for the future. consensus document].
In light of the heterogeneity of NBS programmes in Spain, the group drafted a list of actions detailing what may be required for the diagnosis and treatment of inborn errors of metabolism. To do so, they used as reference a document of the Spanish Ministry of Health, Estrategia en Enfermedades Raras del Sistema Nacional de Salud (Strategy for the Management of Rare Diseases of the National Health System, 2009). Among the listed actions was the need to improve NBS programmes, with a strong recommendation of strengthening the cooperation between ARs and establishing uniform health policies across all of them. All of the above led the Consejo Interterritorial (Interterritorial Council) of the National Health System (NHS) of Spain to create the Working Group for the Development of the Nationwide Health Care Service Portfolio of the NHS in 2012, within which a smaller subset of experts formed a group to select the specific diseases that ought to be included in newborn screening. The definitive proposal was based on reports that were commissioned to the Red de Agencias de Evaluación de Tecnologías Sanitarias (Health Technology Evaluation Agency Network, AETS). In July 2013, in a general assembly, the Consejo Interterritorial approved NBS programmes to test for endocrine and metabolic disorders, which would be thereon included in the nationwide basic services portfolio of the NHS, encouraging the establishment of consensus-based protocols within the framework of the NHS so that screening programmes could be implemented in all ARs in a uniform manner and based on rigorous quality criteria.
The diseases included in the proposed NBS programme, the screening of which, from this moment, became part of the nationwide basic services portfolio offered by the NHS and therefore recommended for inclusion in screening throughout Spain, are 7, out of which 4 are metabolic disorders (*)26:
- 1
CHT.
- 2
Phenylketonuria*
- 3
Cystic fibrosis
- 4
MCADD*
- 5
Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency*
- 6
Glutaric acidaemia type 1*
- 7
Sickle cell disease.
Furthermore, this document mentioned the possibility of expanding the panel to include maple syrup urine disease, isovaleric acidaemia and homocystinuria after trying this option out in a “pilot programme”.
The Consejo Interterritorial also established that the introduction of these NBS programmes in the nationwide services portfolio of the NHS should be accompanied by the development of:
- none-
A newborn screening information system to facilitate the appropriate follow-up and evaluation of newborn screening at the AR and national level.
- none-
A quality assurance system to allow the homogeneous management of all processes in every AR, which would require as a key element the development of consensus-based protocols their implementation in the NHS.
On December 18, 2013, the Consejo Interterritorial of the NHS approved the Objectives and Quality Requirements for programmes for newborn screening of endocrine and metabolic disorders within the NHS. The document detailed the data and indicators that should be included in the newborn screening information system to allow the assessment of the established quality objectives.27 The AETS has continued to carry out cost-effectiveness analyses for the screening of additional diseases that could potentially be included in NBS programmes and determined that screening for biotinidase deficiency is cost-effective,36 while, on the contrary, it has concluded that when it comes to severe combined immunodeficiency (SCID),37 the evidence on the effectiveness of screening is of poor methodological quality, although the disease fulfils many of the criteria required for inclusion in screening programmes. The reports delivered by the AETS involve a long process, and by the time these reviews are published, they no longer reflect the current reality (for instance, the articles reviewed in relation to SCID date from between 2010 and 2016, while the report was published by the AETS in 2018).
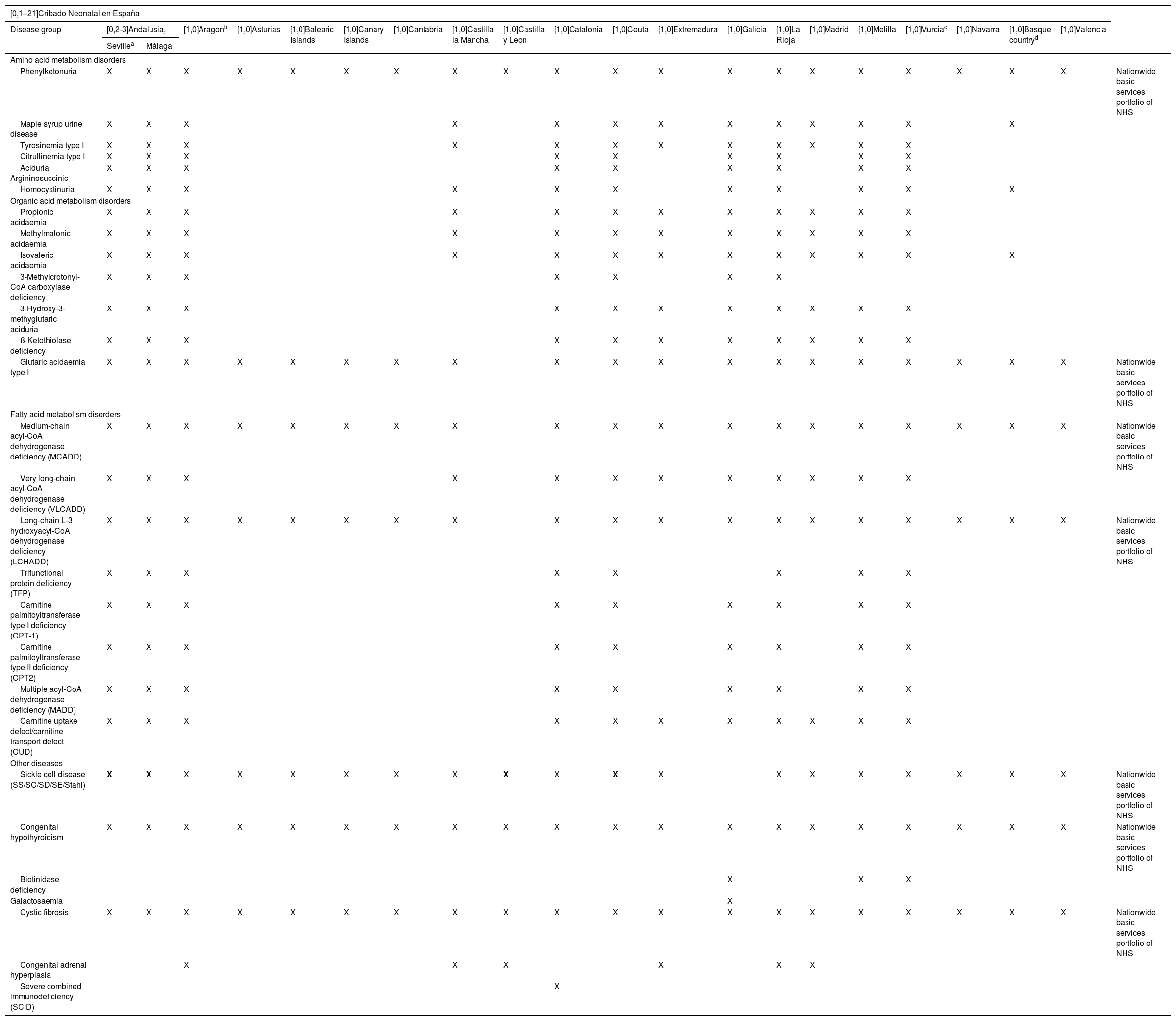
At the time of this writing, all the ARs in Spain adhere to the requisite of including the minimum 7 diseases in their respective NBS programmes except for Galicia, where screening for sickle cell disease is not included, and many ARs, on the basis of current knowledge on the natural course of diseases and advances in medical treatment, screen for many more (Table 4).
Metabolic disorders included in the different newborn screening programmes in Spain.
| [0,1–21]Cribado Neonatal en España | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease group | [0,2-3]Andalusia, | [1,0]Aragonb | [1,0]Asturias | [1,0]Balearic Islands | [1,0]Canary Islands | [1,0]Cantabria | [1,0]Castilla la Mancha | [1,0]Castilla y Leon | [1,0]Catalonia | [1,0]Ceuta | [1,0]Extremadura | [1,0]Galicia | [1,0]La Rioja | [1,0]Madrid | [1,0]Melilla | [1,0]Murciac | [1,0]Navarra | [1,0]Basque countryd | [1,0]Valencia | ||
| Sevillea | Málaga | ||||||||||||||||||||
| Amino acid metabolism disorders | |||||||||||||||||||||
| Phenylketonuria | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | Nationwide basic services portfolio of NHS |
| Maple syrup urine disease | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Tyrosinemia type I | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Citrullinemia type I | X | X | X | X | X | X | X | X | X | ||||||||||||
| Aciduria Argininosuccinic | X | X | X | X | X | X | X | X | X | ||||||||||||
| Homocystinuria | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Organic acid metabolism disorders | |||||||||||||||||||||
| Propionic acidaemia | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Methylmalonic acidaemia | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Isovaleric acidaemia | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| 3-Methylcrotonyl-CoA carboxylase deficiency | X | X | X | X | X | X | X | ||||||||||||||
| 3-Hydroxy-3-methyglutaric aciduria | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| ß-Ketothiolase deficiency | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Glutaric acidaemia type I | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | Nationwide basic services portfolio of NHS | |
| Fatty acid metabolism disorders | |||||||||||||||||||||
| Medium-chain acyl-CoA dehydrogenase deficiency (MCADD) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | Nationwide basic services portfolio of NHS | |
| Very long-chain acyl-CoA dehydrogenase deficiency (VLCADD) | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Long-chain L-3 hydroxyacyl-CoA dehydrogenase deficiency (LCHADD) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | Nationwide basic services portfolio of NHS | |
| Trifunctional protein deficiency (TFP) | X | X | X | X | X | X | X | X | |||||||||||||
| Carnitine palmitoyltransferase type I deficiency (CPT-1) | X | X | X | X | X | X | X | X | X | ||||||||||||
| Carnitine palmitoyltransferase type II deficiency (CPT2) | X | X | X | X | X | X | X | X | X | ||||||||||||
| Multiple acyl-CoA dehydrogenase deficiency (MADD) | X | X | X | X | X | X | X | X | X | ||||||||||||
| Carnitine uptake defect/carnitine transport defect (CUD) | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Other diseases | |||||||||||||||||||||
| Sickle cell disease (SS/SC/SD/SE/Stahl) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | Nationwide basic services portfolio of NHS | |
| Congenital hypothyroidism | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | Nationwide basic services portfolio of NHS |
| Biotinidase deficiency | X | X | X | ||||||||||||||||||
| Galactosaemia | X | ||||||||||||||||||||
| Cystic fibrosis | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | Nationwide basic services portfolio of NHS |
| Congenital adrenal hyperplasia | X | X | X | X | X | X | |||||||||||||||
| Severe combined immunodeficiency (SCID) | X | ||||||||||||||||||||
While the definition of the minimum services that ought to be offered based on clear scientific criteria is a desirable goal, the fact remains that NBS programmes cannot and probably should not be completely uniform, as they must be adapted to the ethnic/genetic factors, social characteristics, health care capacity and economic circumstances of each country or region.
The number of studies published recently on this subject reflects a dynamism that is unprecedented in the history of newborn screening, which will drive an ongoing debate and updating of programmes and guidelines. As the literature on the subject and the evidence on future possibilities in this field continues to develop, the distinction between the concepts of newborn screening and of screening of newborns, which first emerged with the advent of screening by means of MS/MS, is gaining definition and being discussed in detail. Newborn screening reflects an approach to screening that focuses on direct benefits to the newborn, while the concept of screening of newborns goes a little further, taking into account not only newborns but also families and society at large. Spain should not adopt a passive stance in this situation, and both the professionals and the health authorities involved in the different aspects of newborn screening should make an effort to update screening programmes and include any diseases for which advances in medical knowledge and treatment options could lead to improvements in morbidity and mortality outcomes in affected individuals.
Without a doubt, the most important aspect to be debated in the future will be the limits of newborn screening and which aspects we wish to explore and understand in our newborns.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Castiñeras DE, Couce M-L, Marin JL, González-Lamuño D, Rocha H. Situación actual del cribado neonatal de enfermedades metabólicas en España y en el mundo. An Pediatr (Barc). 2019;91:128.