Young children perceive pain as much, or even more than adults, and the pain may have short- and long-term consequences. The literature describes the use of non-pharmacological interventions to alleviate pain during vaccination. The aim of this study was to assess 3 such interventions for analgesia during vaccination: non-nutritive sucking (NNS), breastfeeding (BF), and administration of a 50% dextrose solution (D50W).
Materials and methodsA prospective, non-randomised cohort study was carried out on infants aged 2, 4 and 6 months that received 1, 2, or 3 vaccines, respectively, according to the routine immunisation schedule. There were 3 treatments: NNS, BF, and 2mL of D50W combined with NNS. Pain was assessed using the LLANTO scale, and the duration of crying.
ResultsThe study included 387 infants. The mean scores in the LLANTO scale at ages 2 and 6 months were significantly lower in breastfed infants compared to infants managed with NNS (P=.025 and P<.001, respectively), or infants given D50W (P=.025 and P=.001), and the difference was not statistically significant at age 4 months (P=.21 and P=.27). There were no significant differences between infants managed with NNS and D50W at 2, 4, and 6 months (P=.66, P=.93 and P=.45, respectively). The duration of crying was significantly lower at age 6 months in breastfeed infants compared to infants managed with NNS or D50W (P=.013 and P=.017). No breastfed child (n=129) experienced side effects.
ConclusionsIn infants born to term with adequate weight for gestational age, breastfeeding reduces pain on the administration of 1 or 2 vaccines. When 3 vaccines are given, the reduction is minimal. Administration of D50W does not have any additional analgesic effect in infants compared to being held by a parent combined with NNS during vaccination. BF during vaccination is not associated with any side effects.
Los niños pequeños tienen una percepción del dolor igual o incluso mayor que los adultos, lo que puede tener consecuencias a corto y largo plazo. Se han descrito intervenciones no farmacológicas para aliviar el dolor en los niños durante el acto de la vacunación. El objetivo de este estudio es valorar 3 de estas intervenciones para reducir el dolor asociado a la vacunación: succión no nutritiva (SNN), amamantamiento (LM) y solución de glucosa al 50% (SG50).
Material y métodosEstudio prospectivo, no aleatorizado, de cohortes en niños de 2, 4 y 6 meses que reciben 1, 2 o 3 vacunas, respectivamente, según calendario vacunal sistemático. Se realizaron 3 intervenciones: SNN, LM y ofrecer 2ml de suero glucosado al 50% con SNN. La medición del dolor se efectuó con la escala LLANTO y con el tiempo de llanto.
ResultadosSe incluyó a 387 niños. La media de la escala LLANTO a los 2 y 6 meses era significativamente menor en los niños amamantados que en los niños con SNN (p=0,025 y p<0,001, respectivamente) y en los que recibían SG50 (p=0,025 y p=0,001), sin significación estadística a los 4 meses (p=0,21 y p=0,27). No hubo diferencias significativas entre los niños con SNN y SG50 a los 2, 4 y 6 meses (p=0,66; p=0,93 y p=0,45, respectivamente). El tiempo de llanto fue significativamente menor a los 6 meses en los niños amamantados que en los que recibieron SNN o SG50 (p=0,013 y p=0,017). Ningún niño amamantado (n=129) presentó efectos secundarios.
ConclusionesEn los niños nacidos a término, con peso adecuado a su edad gestacional, el amamantamiento disminuye el dolor cuando se administran 1 y 2 vacunas; cuando se administran 3 vacunas, la disminución es mínima. La administración de SG50 no tiene efecto analgésico adicional respecto a la vacunación de los niños en brazos de sus padres con SNN. La administración de LM durante la vacunación no tiene ningún efecto secundario.
Contrary to the belief held for many years, several studies have demonstrated that infants and young children perceive pain as much or even more than adults, and that pain in this early stages can have short1,2- and long-term consequences (on neurodevelopment and psychosocial development,3,4 on cognitive and learning processes,5 on sleep6 and in adulthood in the form of hyperalgesia1). However, most painful procedures in newborns and infants are still performed without analgesia, mainly due to a lack of training of health care professionals.7,8
Vaccine administration is the painful procedure performed most frequently in the first year of life in healthy infants. The pain it produces could be alleviated with nonpharmacological analgesic methods. Different nonpharmacological interventions have been described in the literature for pain relief in newborns and young children9 that have proven efficacious, inexpensive and well-tolerated. The methods used most frequently are breastfeeding (BF),10 non-nutritive sucking (NNS)11 and administration of sweet solutions.12
Based on this context, we designed a study to assess pain during vaccine administration. Our objective was to determine whether 2 methods of nonpharmacological analgesia (BF and administration of 2mL of a 50% dextrose solution [D50W]) reduced perceived pain compared to the usual practice (NNS with a pacifier/dummy) at ages 2, 4 and 6 months, and to perform a stratified analysis based on the number of administered vaccines.
Materials and methodsWe conducted a prospective, nonrandomised, unmasked cohort study at the Vaccination Centre of the Hospital Casa de Salud (Valencia) between June and December 2016.
The study included infants aged 2, 4 and 6 months brought to the centre to undergo vaccination (official routine immunisation schedule of the Community of Valencia of 2016). We excluded infants brought for vaccination by anyone other than a parent, with abnormal muscle tone or cerebral palsy or respiratory disease; infants born preterm or post-term or small for gestational age; infants that had been given medication for pain relief before vaccine administration, infants with inconsolable crying that could not be soothed before vaccine administration and infants for whom we could not obtain informed consent.
Administered vaccines- -
At 2 months (2 injections): hexavalent diphtheria, tetanus and acellular pertussis vaccine (DTaP) (Infanrix-hexa®) pneumococcal conjugate vaccine (Prevenar 13®).
- -
At 4 months (3 injections): DTaP (Infanrix-hexa®), meningococcal C conjugate vaccine (NeisVac-C®), pneumococcal conjugate vaccine (Prevenar 13®).
- -
At 6 months (1 injection): DTaP (Infanrix-hexa®).
Every vaccine was administered by the same nurse by the intramuscular route (vastus lateralis), inserting the needle at an angle of 70–90° after cleaning the area with physiological saline and delivering the fluid quickly, with no previous aspiration. Second vaccines were inoculated in the same muscle at least 2cm apart from the first injection site. When the patient received 3 vaccines, the third one was injected in the contralateral vastus lateralis. Since the pneumococcal conjugate vaccine is the most painful, it was administered last.13
Intervention- -
A (NNS or control): the mother or father, seated, held the infant in her or his arms and the infant sucked on a pacifier during the administration of the vaccines.
- -
B (BF): the mother was directed to start breastfeeding the infant. The vaccines were administered once the infant was breastfeeding effectively.
- -
C (D50W+NNS): the infant received 2mL of D50W administered orally. After 2min, the scheduled vaccines were administered while the infant sucked on a pacifier in the arms of a parent. We chose to administer dextrose solutions because they are more widely available than sucrose solutions, especially in clinics that do not have a pharmacy department.14 Furthermore, sucrose can produce side effects on children with fructose intolerance.15 Adverse reactions have not been reported in association with the use of dextrose solutions with the exception of a few episodes of bradycardia and oxygen desaturation in very preterm newborns that were clinically insignificant.14 We chose a 50% concentration because recently published data suggest that lower concentrations may not be effective in infants past the neonatal period.16–18
We classified the infants into 3 groups according to age: group 2 (2 months), group 4 (4 months) and group 6 (6 months). We allocated participants to the different interventions based on their usual feeding modality: breastfed infants were placed in subgroups 2 B, 4 B or 6 B. We allocated infants that were not breastfed in groups of 8 consecutive participants to either NNS (2 A, 4 A, 6 A) or D50W (2 C, 4 C, 6 C) in alternating order.
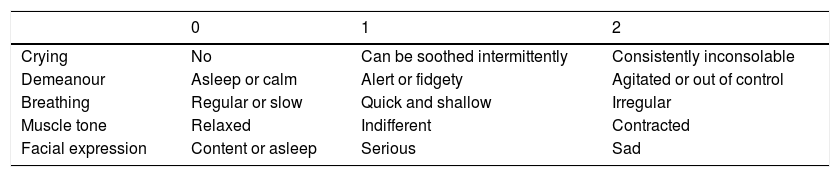
Measurement tools(A) LLANTO scale,19 applied at the peak of crying intensity, always by the same physician (a paediatrics specialist). The severity of pain was calculated by adding the individual scores given to 5 items on a scale from 0 to 2 (crying, demeanour, breathing, posture, facial expression). Thus, the possible total scores ranged between 0 (no pain) and 10 (maximum pain) (Table 1). This scale was designed for the assessment of infants and preschool-aged children at the Hospital Universitario La Paz (Madrid), so it is adapted to our language and culture. It does not require the use of any electronic devices (pulse oximeter, sphygmomanometer) and allows assessment from afar, which reduces the potential interference derived from the presence of the evaluator. It can be used to accurately estimate the severity of pain in 20–40s, it has uniform application criteria and it is highly objective. The results obtained with this scale correlate strongly to those obtained with the CHEOPS scale,20 which is considered the gold standard for the assessment of pain in children.
LLANTO scale.
| 0 | 1 | 2 | |
|---|---|---|---|
| Crying | No | Can be soothed intermittently | Consistently inconsolable |
| Demeanour | Asleep or calm | Alert or fidgety | Agitated or out of control |
| Breathing | Regular or slow | Quick and shallow | Irregular |
| Muscle tone | Relaxed | Indifferent | Contracted |
| Facial expression | Content or asleep | Serious | Sad |
Source: Reinoso-Barbero et al.19
(B) Duration of crying: measured in seconds from the start of crying after injection of the first vaccine until the infant was quiet for 5s, excluding these 5s (maximum time measured: 3min). The advantage of the duration of crying is that it can be measured objectively, its disadvantage is that it may be determined by causes other than pain (hunger, thirst, behaviour of parents and health care staff …).
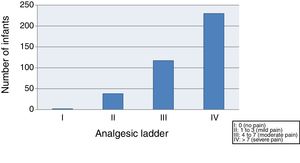
(C) Based on the score in the LLANTO scale, we classified pain as absent, mild, moderate or severe, conforming to the analgesic ladder of the World Health Organization (WHO):
- -
I: 0 (absent)
- -
II: 1–3 (mild)
- -
III: 4–7 (moderate)
- -
IV: >7 (severe)
We estimated that including 43 patients in each subgroup would allow the detection of a significant difference of 0.85 points in the LLANTO scale. We assumed a standard deviation from the mean of 1.2 points, and calculated the sample size for a power of 90% and a probability of type I error of 5%. We defined statistical significance as a P-value of less than 0.05.
Data collectionWe collected clinical and sociodemographic data: sex, age and educational attainment of parents, social class, whether the infant was accompanied by one or both parents, number of siblings and birth order of the participant, gestational age and birth weight, Apgar score and whether the infant was awake or asleep at the time vaccination started. We obtained this information by surveying parents, collecting the data in a form created for the purpose and subsequently entering it in an Excel spreadsheet for analysis. We categorised social class using the scheme proposed by Domingo et al.,21 modified to divide parents into 2 groups: class I to II (higher professional and managerial occupations, higher- and middle-grade technicians) and class III to VII (intermediate occupations, administrative workers, manual labour, unskilled labour and others).
Statistical analysisWe used the Kolmogorov–Smirnov test to assess the normality of the distribution in all variables. Based on whether the distribution was or not normal, we compared quantitative variables by means of the Student's t test (2 variables), analysis of variance (3 or more variables), Mann–Whitney U test (2 variables) or Kruskal–Wallis test (3 or more variables). We used the χ2 test to compare qualitative variables.
Ethical and legal considerationsThe study was approved by the Ethics Committee of the Hospital Universitario y Politécnico La Fe (Valencia). Parents agreed to participation in the study by signing an informed consent form.
ResultsThe study included 387 infants (129 in each age group). Each subgroup consisted of 43 infants: 197 male (50.9%) and 190 female (49.1%); 189 (48.8%) were the first-born child, 175 (45.2%) the second-born and the rest had a birth order of third or higher. The 1-minute Apgar score was greater than 7 in 363 infants (93.8%), and the 5-minute Apgar score was 8 or greater in all participants. The mean maternal age was 34.3 years (23–50) and the mean paternal age was 36.5 (25–53). In our sample, 57.7% of families belonged to social classes I or II, with a percentage of mothers with a university education of 68% and of fathers with a university education of 57.1%. In 58.1% of cases, only one of the parents accompanied the infant to the vaccination appointment, while both parents attended in 41.9% of cases. At the time vaccination started, 363 of the infants (93.8%) were awake, while the remaining 24 were asleep.
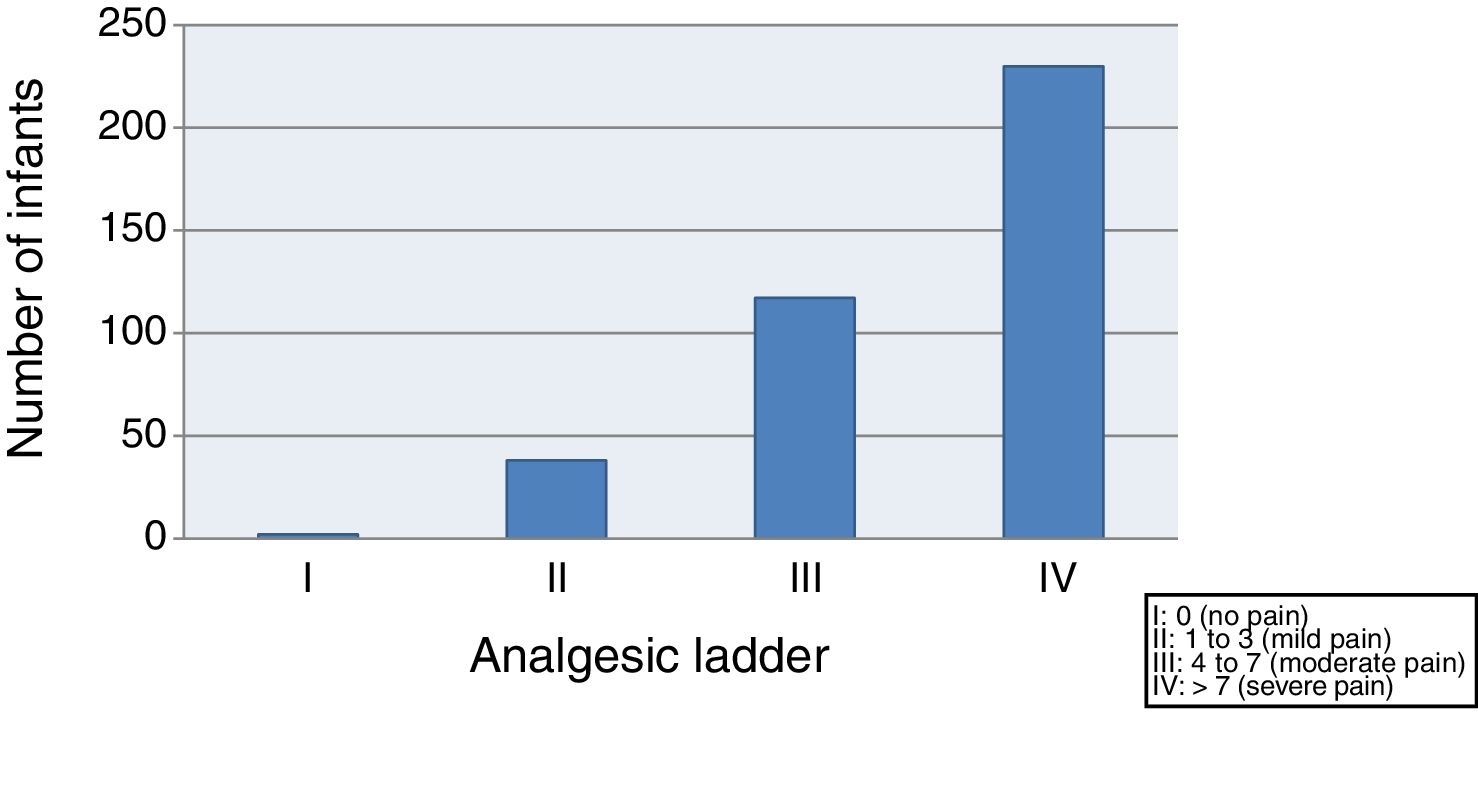
We started by evaluating the severity of pain, finding that 59.4% of the infants suffered moderate to severe pain (class III–IV) after vaccination, and only 10.3% exhibited mild pain or no pain (class I–II) (Fig. 1).
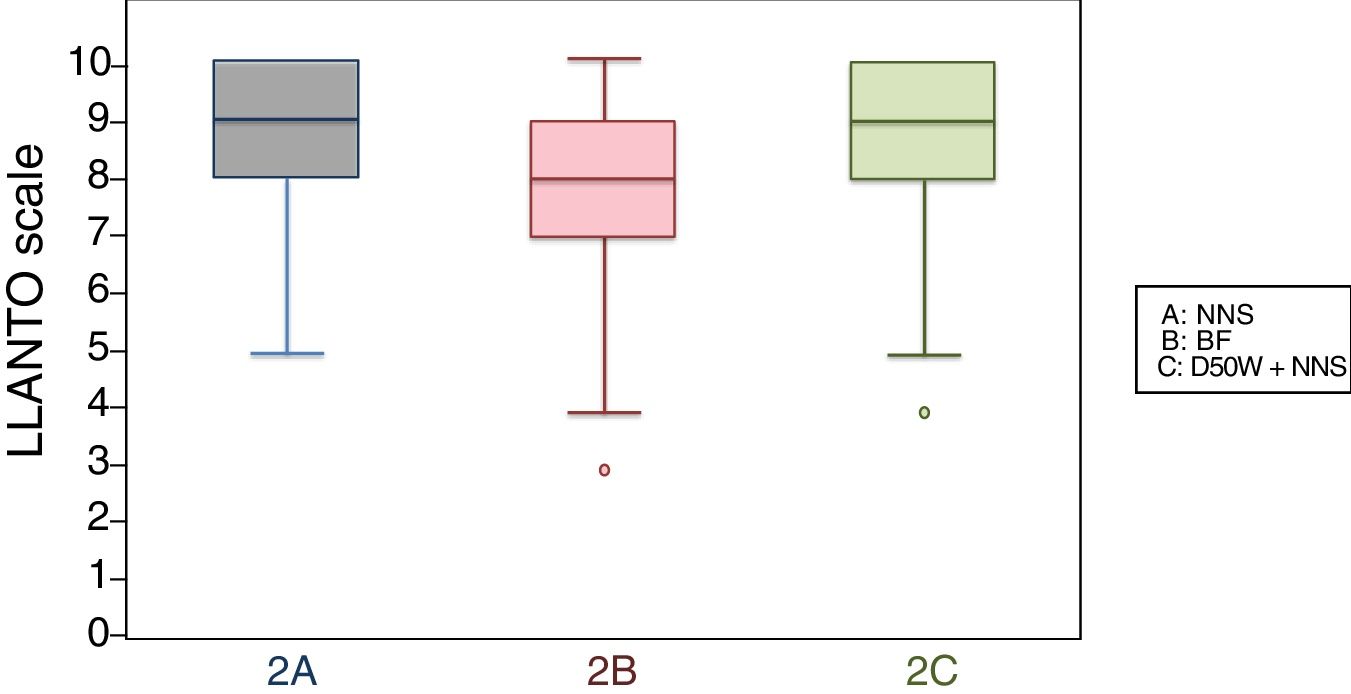
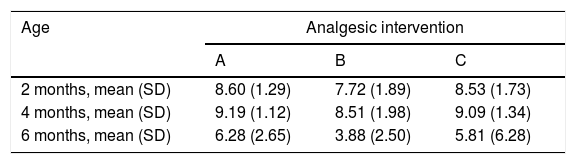
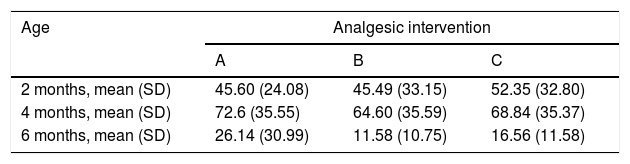
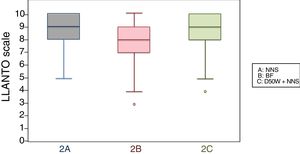
When we classified infants in this age group according to the analgesic ladder, we found that the proportion of infants breastfed during vaccination that experienced severe pain was smaller compared to the other subgroups, and that there was a higher proportion that experienced moderate pain. There were also 2 infants in the BF group that exhibited mild pain. We found a significantly lower mean score in the LLANTO scale in infants managed with BF compared to infants that received D50W (P=.024) and infants in the control group (P=.025). We did not find significant differences between the subgroup that received D50W and the control group (P=.66) (Table 2). As can be seen in Fig. 2, the median in breastfed infants was lower compared to the other 2 subgroups. We did not find significant differences in the duration of crying between groups (Table 3).
LLANTO scores (mean±SD).
| Age | Analgesic intervention | ||
|---|---|---|---|
| A | B | C | |
| 2 months, mean (SD) | 8.60 (1.29) | 7.72 (1.89) | 8.53 (1.73) |
| 4 months, mean (SD) | 9.19 (1.12) | 8.51 (1.98) | 9.09 (1.34) |
| 6 months, mean (SD) | 6.28 (2.65) | 3.88 (2.50) | 5.81 (6.28) |
Analgesic interventions: A, non-nutritive sucking (NNS); B, breastfeeding; C, 50% dextrose solution+NNS.
Duration of crying (mean±SD in seconds).
| Age | Analgesic intervention | ||
|---|---|---|---|
| A | B | C | |
| 2 months, mean (SD) | 45.60 (24.08) | 45.49 (33.15) | 52.35 (32.80) |
| 4 months, mean (SD) | 72.6 (35.55) | 64.60 (35.59) | 68.84 (35.37) |
| 6 months, mean (SD) | 26.14 (30.99) | 11.58 (10.75) | 16.56 (11.58) |
Analgesic interventions: A, non-nutritive sucking (NNS); B, breastfeeding; C, 50% dextrose solution+NNS.
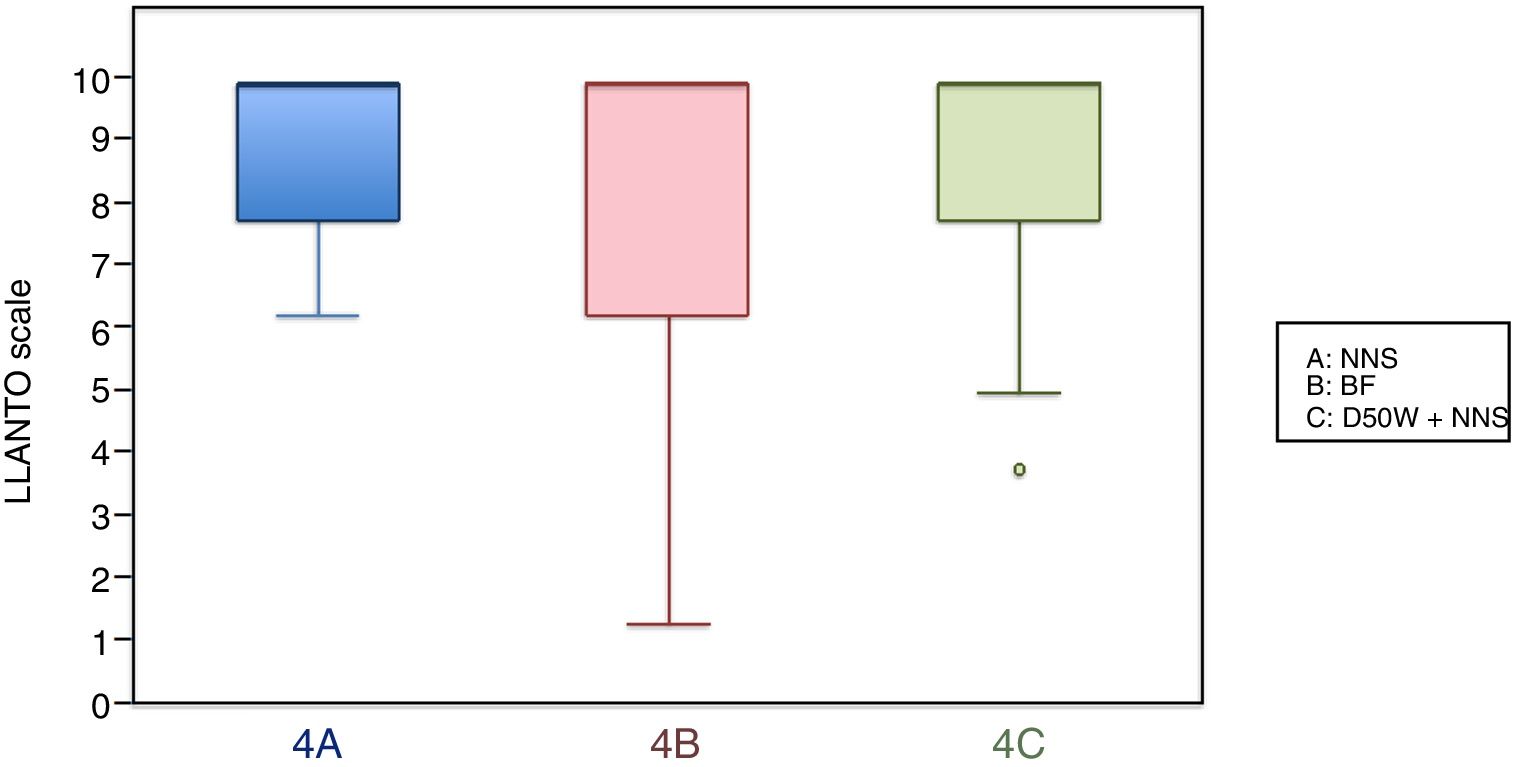
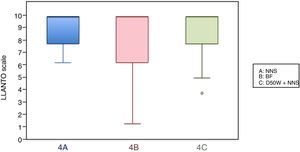
In this age group, the mean LLANTO scale score was lowest in the BF subgroup, although the differences were not statistically significant: 4 A vs 4 B (P=.21), 4 A vs 4 C (P=.93) and 4 B vs 4 C (P=.27) (Table 2). The median score was 10 in the 3 subgroups (Fig. 3). We did not find statistically significant differences in the duration of crying between the subgroups (Table 3).
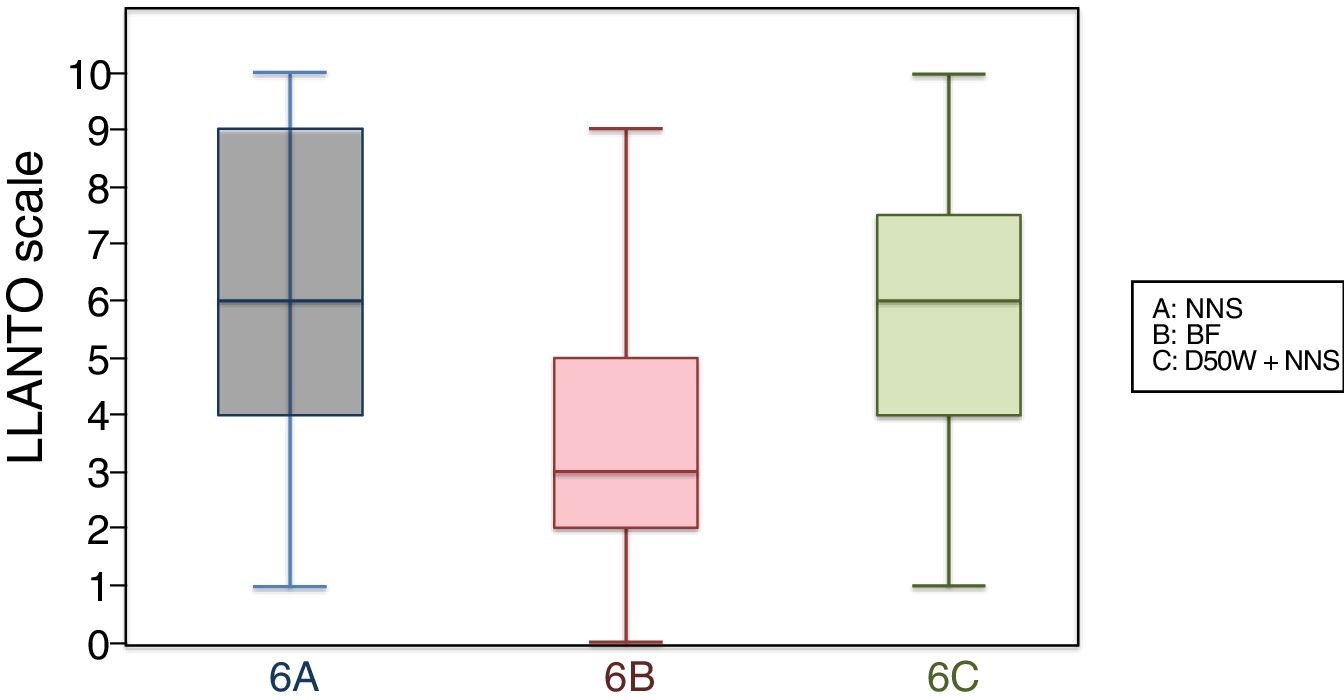
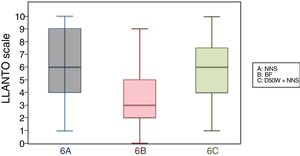
6 months (1 injection)When we classified patients according to the analgesic ladder, we found that 22 breastfed infants (nearly 50%) exhibited no pain or mild pain compared to 10 in the D50W subgroup and 5 in the control group. Pain was moderate or severe more frequently in the control and D50W subgroups. We found a lower score in the LLANTO scale in the BF subgroup compared to the D50W subgroup (P=.001) and controls (P<.001) and found no differences between the 6 A and 6 C subgroups (P=.45) (Table 2). As seen in Fig. 4, the median score in the BF subgroup was lower compared to the other 2 subgroups. We also found a shorter duration of crying in infants that were breastfed, a difference that was statistically significant between subgroups 6 B and 6 A (P=.013) and subgroups 6 B and 6 C (P=.017). We found no significant differences between subgroups 6 A and 6 C (P=.66) (Table 3).
Comparison of other variablesWhen we compared boys and girls, we found no significant differences at 2 and 4 months (P>.05). We did find a significantly higher score in the LLANTO scale and an increased duration of crying in girls compared to boys at 6 months (P=.026 in both).
We did not find differences based on birth order, maternal age (less than/greater than 40 years), socioeconomic status or whether the infant was accompanied by one or both parents.
We did not make a comparison based on whether infants were awake or asleep at the start of vaccination, as only 24 infants were initially asleep.
Adverse eventsNone of the infants treated with BF (n=129) experienced adverse events. Of the 129 that received D50W, 3 experienced mild choking that resolved spontaneously.
DiscussionDifferent studies have proven that nonpharmacological analgesic interventions can reduce pain resulting from minor procedures in newborns.12,14,22,23 However, there is no conclusive evidence on the subject in infants past the neonatal period.16,17
When we analysed the pain that developed in infants aged 2, 4 and 6 months at the time of vaccination, we found that 90% experienced moderate or severe pain according to the LLANTO scale. The implementation of simple and harmless analgesic interventions with participation of the parents during vaccination could help lessen the resistance to vaccinate that exists in part of our population.
The first study that assessed the efficacy of BF for analgesia was published in 2002.24 By now there is ample evidence of its effectiveness for pain relief in newborns undergoing a single painful procedure,23 with an effect that is stronger compared to oral administration of sweet solutions.25,26 There are far fewer studies on the subject in infants, and they are also quite heterogeneous. In our study, we found that when only 1 vaccine was administered (age 6 months), infants that were breastfeed had significantly lower LLANTO scale scores and durations of crying compared to infants given D50W and controls, which was consistent with previous studies.16,27 In infants that received 2 injections (age 2 months), we found a significant decrease in the LLANTO scale score in the BF subgroup compared to the other subgroups, but not in the duration of crying. In infants that received 3 vaccines (age 4 months), while we observed a reduction in the LLANTO scale score and the duration of crying in the BF subgroup, the differences were not statistically significant, which suggests that BF is not sufficient for analgesia in the context of an intense painful stimulus. The few studies that have analysed pain associated with vaccination did not compare results based on the number of vaccines administered,28,29 which makes our study the first to assess pain in relation to the number of painful stimuli experienced by the participants.
Many studies have assessed the use of different dextrose and sucrose solutions at different concentrations during the neonatal period, confirming that they can effectively reduce the pain associated with painful minor procedures (heel puncture, venous puncture, vaccination).12,30,31 The studies on the effects of sweet solutions during vaccination in infants aged 1–12 months generally show a lower efficacy compared to newborns. In our study, we did not find any significant differences in the LLANTO scale or the duration of crying between the D50W and the control groups, which was consistent with the reviewed literature.17 A study conducted in Spain did find a statistically significant reduction in the duration of crying with the administration of a 75% sucrose solution during the administration of a single vaccine, although this effect was considered irrelevant from a clinical standpoint.32 In contrast, other studies have found evidence of significant pain relief with administration of dextrose or sucrose solutions, usually at high concentrations.33 The disparity between results could be due to methodological differences between the studies (means of administration and concentrations of sweet solutions, vaccines administered, injection technique and possible use of additional analgesic methods, such as NNS or vaccination while infant is held in a parent's arms). In order to determine the actual effectiveness of sweet solutions, in our study all infants were held by a parent and used NNS. The soothing effect of NNS has been confirmed by a few studies in newborns and seems to be associated with the number of sucks per minute.31,34,35 Similarly, other studies have found evidence of the analgesic effect of being held by the mother.11 Our results suggest that administration of D50W does not add to the analgesic effect achieved with NNS while the infant is held by a parent during vaccination at 2, 4 and 6 months, which is consistent with the previous literature,25 although some studies conducted during the neonatal period have found a synergistic effect of the administration of sweet solutions in combination with NNS in newborns.31
Some studies in adults have found that pain responses vary based on cultural background and socioeconomic level, of which we found no evidence in our study.36 We did not find differences in pain perception based on sex at 2 and 4 months. However, at 6 months we did find a more severe pain response in girls. When it comes to the adult population, several studies have found evidence of a greater pain response in female versus male participants.37 In children, the findings are heterogeneous, and while some studies have found no differences between the sexes,38 others have found more severe pain responses in girls.39,40 Further research is needed to confirm behavioural differences in the response to pain between the sexes.
None of the patients managed with BF in our study exhibited side effects, which is consistent with the literature.27 Of the patients managed with D50W, only 3 experienced mild choking, from which they recovered spontaneously with no clinical repercussions. These findings were consistent with the reviewed literature.14,22,33
Having mothers breastfeed infants during the administration of vaccines is an effective analgesic intervention that can also encourage mothers to breastfeed their children, which facilitates bonding and does not add to the costs of the health care system.23 In addition, it does not require any form of storage or special materials. At present, based on our results, we consider that no infant should receive the vaccinations at 2, 4 and 6 months without first informing the mother that she can breastfeed the infant, as this intervention would reduce the pain that the infant feels during vaccination.
Our findings showed that D50W was not effective for analgesia during vaccination of infants aged 2, 4 and 6 months, as the sweet solution did not have a synergistic effect in combination with NNS.
There are several limitations to our study, among them that it was not blinded, as allocation to subgroups was dependent on ongoing breastfeeding; the overall high socioeconomic level could be a source of bias, and allowing parents to talk to and caress their children during vaccination could also have influenced the results. We also ought to mention that since we excluded infants born preterm or small for gestational age, further research should be made in these subpopulations to confirm our findings.
ConclusionsIn infants born at term with a weight appropriate for gestational age, BF had an analgesic effect during the administration of 1 or 2 vaccines; when 3 vaccines were administered, the pain relief was negligible. On the other hand, administration of D50W did not seem to have an analgesic effect that added to the effect of NNS while the infant was being held by a parent during vaccination. We cannot rule out the possibility that other sweet solutions or solutions at other concentrations could be effective for analgesia. We believe we ought to underscore the fact that BF during vaccination has no side effects.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all our colleagues in the Department of Paediatrics of Hospital Casa de Salud in Valencia for their collaboration in the study; as well as all the parents that agreed to participate in the study and gave consent for the participation of their children.
Please cite this article as: Nieto García A, Berbel Tornero O, Monleón Sancho J, Alberola-Rubio J, López Rubio ME, Picó Sirvent L. Evaluación del dolor en niños de 2, 4 y 6 meses tras la aplicación de métodos de analgesia no farmacológica durante la vacunación. An Pediatr (Barc). 2019;91:73–79.