Synovial fluid (SF) analysis is an important tool for the diagnosis of patients with juvenile idiopathic arthritis (JIA).
Patients and methodsA retrospective analysis was carried out of cytological features of SF samples obtained from patients with JIA during the period 2008–2016.
ResultsA total of 102 SF samples from 59 patients were analysed. JIA was more common in females (66%). The most frequent form was persistent oligoarticular JIA (52.5%). The median age at onset was 5 years (IQR 2.4–11.8). SF usually showed an inflammatory pattern (median white blood cells count 11,757/mm3; IQR 4,543–18,800), with a predominance of polymorphonuclear (PMN) cells (61%; IQR 30–75). Eight patients (14%) had white blood cells counts of less than 2000cells/mm3, with predominance of mononuclear cells (80%), whereas 3 patients (5%) had white blood cells counts higher than 50,000cells/mm3, with a predominance of PMN cells (90%). Synovial white blood cells count did not show significant differences among the different forms of JIA. The median synovial white blood cells count in ANA-positive patients was 20% lower than in ANA-negative (9,340 vs. 11,600/mm3; P=.23). The proportion of PMN increased with increasing levels of ESR (P<.001) and/or CRP (P=.03). No significant correlation was found between JADAS-10 and synovial white blood cells count (P=.4). SF obtained from different joints in simultaneous arthrocentesis showed a significant correlation P=.001).
ConclusionSF from JIA patients usually had inflammatory characteristics, although 19% of the patients showed white blood cells counts below 2000cells/mm3 or higher than 50,000cells/mm3. SF cell count was non-significantly lower in ANA-positive patients, and the proportion of PMN increased with increasing levels of ESR/CRP.
El análisis del líquido sinovial (LS) es una herramienta importante en el diagnóstico de pacientes con artritis idiopática juvenil (AIJ).
Pacientes y métodosAnálisis retrospectivo de las características citológicas del LS obtenido de pacientes con AIJ en el periodo 2008-2016.
ResultadosSe analizaron 102 LS de 59 pacientes. El 66% fueron mujeres y la forma clínica más frecuente fue la AIJ oligoarticular persistente (52,5%). La mediana de edad al inicio fue de 5 años (RIC 2,4-11,8). El LS generalmente era de características inflamatorias (mediana leucocitos 11.757/mm3; RIC 4.543-18.800) con predominio de polimorfonucleares (PMN, 61%; RIC 30-75). Ocho pacientes (14%) presentaron recuentos inferiores a 2.000cél/mm3, con predominio de mononucleares (80%), mientras que 3 pacientes (5%) presentaron recuentos superiores a 50.000cél/mm3, con predominio de PMN (90%). No se encontraron diferencias en los recuentos celulares entre las distintas formas de AIJ. La mediana del recuento de leucocitos de pacientes positivos para ANA fue un 20% inferior a la de niños negativos para ANA (9.340 vs. 11.600/mm3; p=0,23). La proporción de PMN en LS tendía a aumentar conforme se incrementaba la VSG (p<0,001) y/o la PCR (p=0,03). No existe correlación del índice JADAS-10 con el recuento en LS (p=0,4). El LS en artrocentesis simultáneas de diferentes articulaciones mostró una correlación significativa (p=0,001).
ConclusionesEl LS de pacientes con AIJ generalmente tiene características inflamatorias, aunque un 19% presentó recuentos inferiores a 2.000 o superiores a 50.000cél/mm3. Los recuentos en pacientes positivos para ANA tendían a ser menores que en los negativos para ANA (no significativo). La proporción de PMN aumentaba con los reactantes.
Juvenile idiopathic arthritis (JIA) is the most frequent chronic rheumatic disease in paediatrics. The term JIA encompasses all forms of chronic arthritis in the paediatric age group and is therefore a heterogeneous group of diseases with different clinical and immunogenetic characteristics. Thus, there are also differences in the treatment, prognosis and long-term outcomes of the various clinical forms of JIA.1,2
In 1994, the International League of Associations for Rheumatology proposed new criteria for the classification of paediatric arthritis to avoid the confusion generated by the use of different criteria to refer to the same disease on either side of the Atlantic (juvenile chronic arthritis, juvenile rheumatoid arthritis), coining the new term JIA. These criteria have been reviewed twice since, most recently in 2001.3 The International League of Associations for Rheumatology established 7 categories: systemic-onset arthritis, oligoarticular (involvement of 4 or fewer joints), polyarticular (inflammation of at least 5 joints, further classified based on the presence of rheumatoid factor [RF] as RF-positive or RF-negative), psoriatic arthritis, enthesitis-related arthritis (ERA) and undifferentiated arthritis.
Synovial fluid (SF) analysis, and cell counts in particular, are a powerful tool for the characterisation of arthritis, as they allow discrimination of inflammatory versus non-inflammatory processes based on the total and differential white blood cell (WBC) counts.4,5 Nevertheless, patients with JIA may exhibit a degree of variability in the characteristics of SF based on the number of affected joints.6
The aim of our study was to define the cytological characteristics of SF in patients with a diagnosis of JIA. We also analysed the differences in these characteristics: (1) based on the number of affected joints; (2) between fluid samples obtained in the same date from different joints in a single patient, and (3) between SF samples obtained in different dates in the same patient.
Sample and methodsWe conducted a retrospective, cross-sectional observational descriptive study of all patients given a diagnosis of JIA at the Hospital Universitario 12 de Octubre in whom sufficient SF was obtained at the time of diagnosis or during relapses allowing a complete cytological investigation between March 1, 2008 and February 28, 2016. Juvenile idiopathic arthritis was diagnosed based on the criteria established by the International League of Associations for Rheumatology.3,7
We reviewed the health records of these patients, collecting demographic data (sex, age at onset), clinical data (clinical form of JIA, number of affected joints, course of disease, assessment of disease by physician and the family) and laboratory data (presence of antinuclear antibodies [ANA], RF and antigen HLA-B27, acute phase reactants and characteristics of SF). Using this information, we calculated the Juvenile Arthritis Disease Activity Score 10 (JADAS-10), a brief index of disease activity that takes into account the number of affected joints, the general assessment of disease activity by the physician measured by means of a visual analogue scale from 0 to 10, the general assessment of disease activity by the parents measured by means of a visual analogue scale from 0 to 10, and the erythrocyte sedimentation rate (ESR).8,9
When it came to SF analysis, all samples with a volume of at least 2mL were submitted to the laboratory for performance of cell counts, biochemical analysis and, when considered indicated, microbiological testing. The sample for cytological analysis was submitted in a test tube containing ethylenediaminetetraacetic acid, and cell counts were performed within a few hours to prevent degenerative changes in sample cells. In patients in whom lesser volumes of fluid were obtained, who were mainly those with monoarthritis in the ankle, wrist or elbow, samples were submitted for microbiological testing only.
For all samples of SF, we collected the cytological characteristics (total WBC count, percentage of polymorphonuclear [PMN] cells and of mononuclear [MN] cells) and the concentrations of glucose and protein.
We classified SF based on the criteria established by the American College of Rheumatology: normal, WBC greater than 200/mm3; non-inflammatory or traumatic, WBC between 200 and 2000/mm3; inflammatory, WBC between 2000 and 50000/mm3, and septic, WBC greater than 50000/mm3.10,11
We performed a descriptive analysis of the data, summarising quantitative variables as absolute values, median and interquartile range (IQR), and categorical variables as absolute frequencies and percentages. We assessed the normality of the distribution of quantitative data by means of graphic representation and the Shapiro–Wilks test. To analyse differences in quantitative variables between groups, we used the Student's t test or the Mann–Whitney U test based on whether the distribution was or not normal. We explored the association between quantitative variables by calculating the Pearson or the Spearman correlation coefficient based on whether the data distribution was or not normal and the graphic analysis of the data. We performed the statistical analyses with the software packages STATA 12.1 (StataCorp, Texas, USA) and SPSS version 22.0 (SPSS Inc., IBM, USA).
ResultsDemographic characteristics of the sampleDuring the period under study, at total of 102 eligible samples of SF were obtained from 59 patients with a diagnosis of JIA.
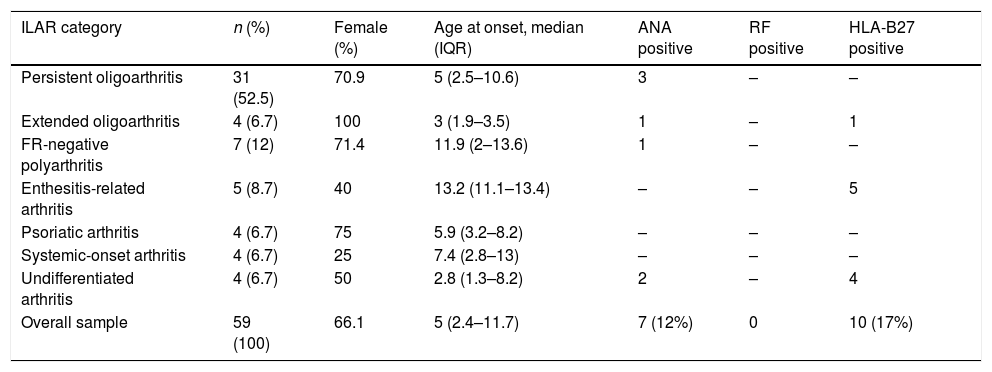
The disease was more frequent in female patients (39/59; 66%), except in the systemic-onset and ERA forms (Table 1). The median age at onset was 5 years (IQR, 2.4–11.8) and the median age at diagnosis was 5.1 years (IQR, 2.6–12.1). The median age of onset was youngest in patients with extended oligoarticular disease followed by patients with undifferentiated arthritis; on the other hand, the latest onset corresponded to ERA.
Demographic and laboratory characteristics of patients in the sample by type of juvenile idiopathic arthritis.
| ILAR category | n (%) | Female (%) | Age at onset, median (IQR) | ANA positive | RF positive | HLA-B27 positive |
|---|---|---|---|---|---|---|
| Persistent oligoarthritis | 31 (52.5) | 70.9 | 5 (2.5–10.6) | 3 | – | – |
| Extended oligoarthritis | 4 (6.7) | 100 | 3 (1.9–3.5) | 1 | – | 1 |
| FR-negative polyarthritis | 7 (12) | 71.4 | 11.9 (2–13.6) | 1 | – | – |
| Enthesitis-related arthritis | 5 (8.7) | 40 | 13.2 (11.1–13.4) | – | – | 5 |
| Psoriatic arthritis | 4 (6.7) | 75 | 5.9 (3.2–8.2) | – | – | – |
| Systemic-onset arthritis | 4 (6.7) | 25 | 7.4 (2.8–13) | – | – | – |
| Undifferentiated arthritis | 4 (6.7) | 50 | 2.8 (1.3–8.2) | 2 | – | 4 |
| Overall sample | 59 (100) | 66.1 | 5 (2.4–11.7) | 7 (12%) | 0 | 10 (17%) |
The most frequent form of JIA was persistent oligoarthritis (n=31; 52.5%), followed by RF-negative polyarthritis (n=7; 12%). Seven patients (12%) had chronic anterior uveitis (Table 1).
A total of 7 patients (12%) had ANA in serum with titres of 1:160 or greater in 2 samples obtained at least 12 weeks apart; all of these patients were aged less than 6 years (median, 24 months; IQR, 18–29). Another 10 patients tested positive for HLA-B27 antigen, of who 5 had ERA (Table 1).
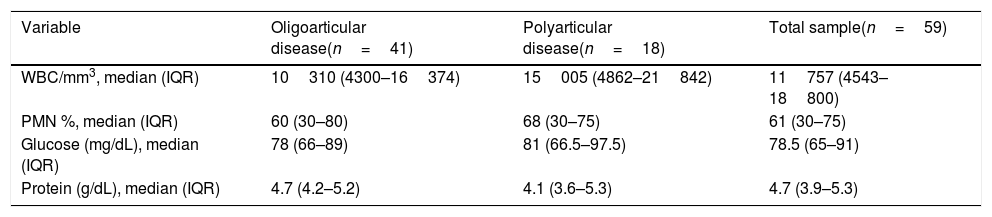
Cytological characteristics of synovial fluid in the total sample and by clinical formThe median WBC count in the SF of patients with JIA was of 11757/mm3 (IQR, 4543–18800) with a median percentage of PMN of 61% (IQR, 30–75). The chemistry tests revealed a median glucose concentration of 78.5mg/dL (IQR, 65–91) and a median protein concentration of 4.7g/dL (IQR, 3.9–5.28).
Eight of the 59 patients (14%) had a WBC count of less than 2000cells/mm3 in the initial arthrocentesis (median, 739; IQR, 374–1001). The counts corresponded to oligoarthritis in 5, polyarthritis in 2 and ERA in 1. In 7 out of the 8, the percentage of MN cells was greater than 75%. All of these patients underwent a Mantoux test, with an induration of 0mm at 72h, and samples of SF were submitted to the laboratory for an acid-fast stain and culture for anaerobic and aerobic bacteria and mycobacteria, with no growth in any of the cultures.
On the other end of the spectrum, 3 patients (5%) had WBC counts of more than 50000/mm3 at the time of diagnosis, with a clear predominance of PMN cells (90%) in all. Each of these patients had a different form of JIA: systemic-onset JIA with absence of systemic symptoms but a very severe persistent polyarthritis, ERA in a male patient that tested positive for HLA-B27 and had polyarticular involvement, and oligoarticular JIA. We ought to highlight that at the time of the arthrocentesis all 3 patients exhibited a marked elevation of acute phase reactants (C-reactive protein [CRP] 12–25mg/dL, ESR, 74–120mm).
When we analysed SF characteristics based on the clinical form of JIA (Table 2), we found that patients with oligoarthritis had lower WBC counts (median, 10310/mm3) compared to patients with polyarthritis, in whom the median count was of 15005/mm3, although this difference was not statistically significant. We also found no significant differences in the PMN/MN ratio (Table 2), which was 2 in the oligoarticular JIA group and 2.1 in the polyarticular JIA group.
Cytological characteristics of synovial fluid by number of involved joints and in the total sample.
| Variable | Oligoarticular disease(n=41) | Polyarticular disease(n=18) | Total sample(n=59) |
|---|---|---|---|
| WBC/mm3, median (IQR) | 10310 (4300–16374) | 15005 (4862–21842) | 11757 (4543–18800) |
| PMN %, median (IQR) | 60 (30–80) | 68 (30–75) | 61 (30–75) |
| Glucose (mg/dL), median (IQR) | 78 (66–89) | 81 (66.5–97.5) | 78.5 (65–91) |
| Protein (g/dL), median (IQR) | 4.7 (4.2–5.2) | 4.1 (3.6–5.3) | 4.7 (3.9–5.3) |
Patients with oligoarticular disease had arthritis in 4 or fewer joints, those with polyarticular disease had arthritis in 5 or more joints.
Patients with arthritis that tested negative for ANA had WBC counts in SF that were 20% greater compared to those of ANA-positive patients ANA (median, 11600/mm3 [IQR, 4340–20573] vs 9340/mm3 [IQR, 6840–12694]), although this difference was not statistically significant. We also found no differences in the PMN/MN ratio based on the results of the ANA test.
When it came to the presence of HLA-B27 antigen, WBC counts were 50% greater in patients that tested positive for HLA-B27 (18841/mm3) compared to those that tested negative (10253/mm3), although these differences, for instance in the PMN/MN ratio (2.9 vs 1.7, respectively), were not statistically significant.
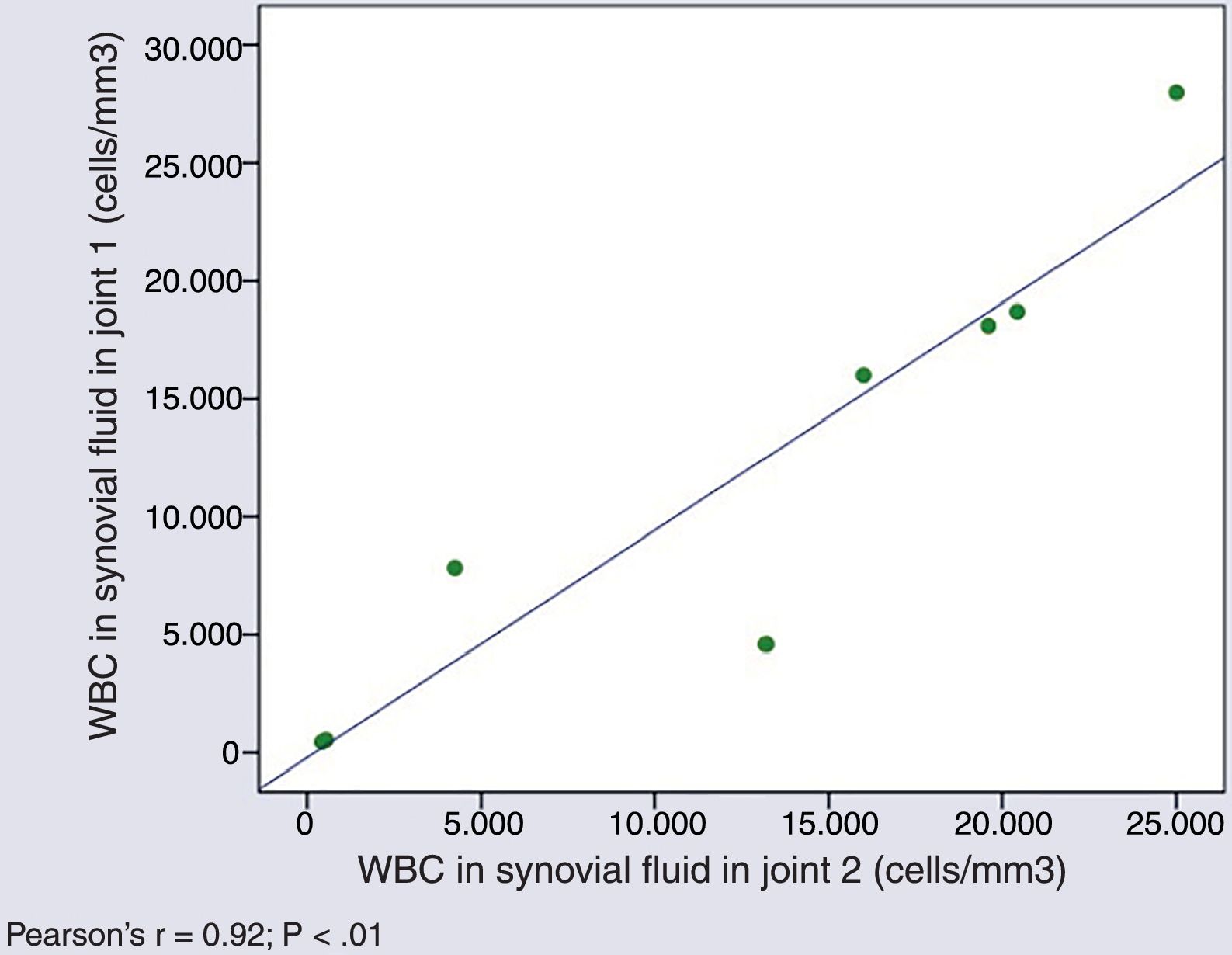
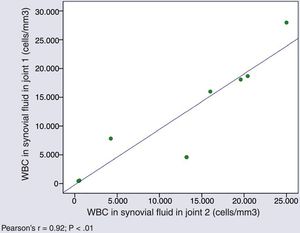
Cytological characteristics of synovial fluid in simultaneous arthrocentesis samplesThe WBC counts in SF samples obtained from different joints of a same patient in a single day were very similar (Pearson's r=0.927; P=.001) (Fig. 1).
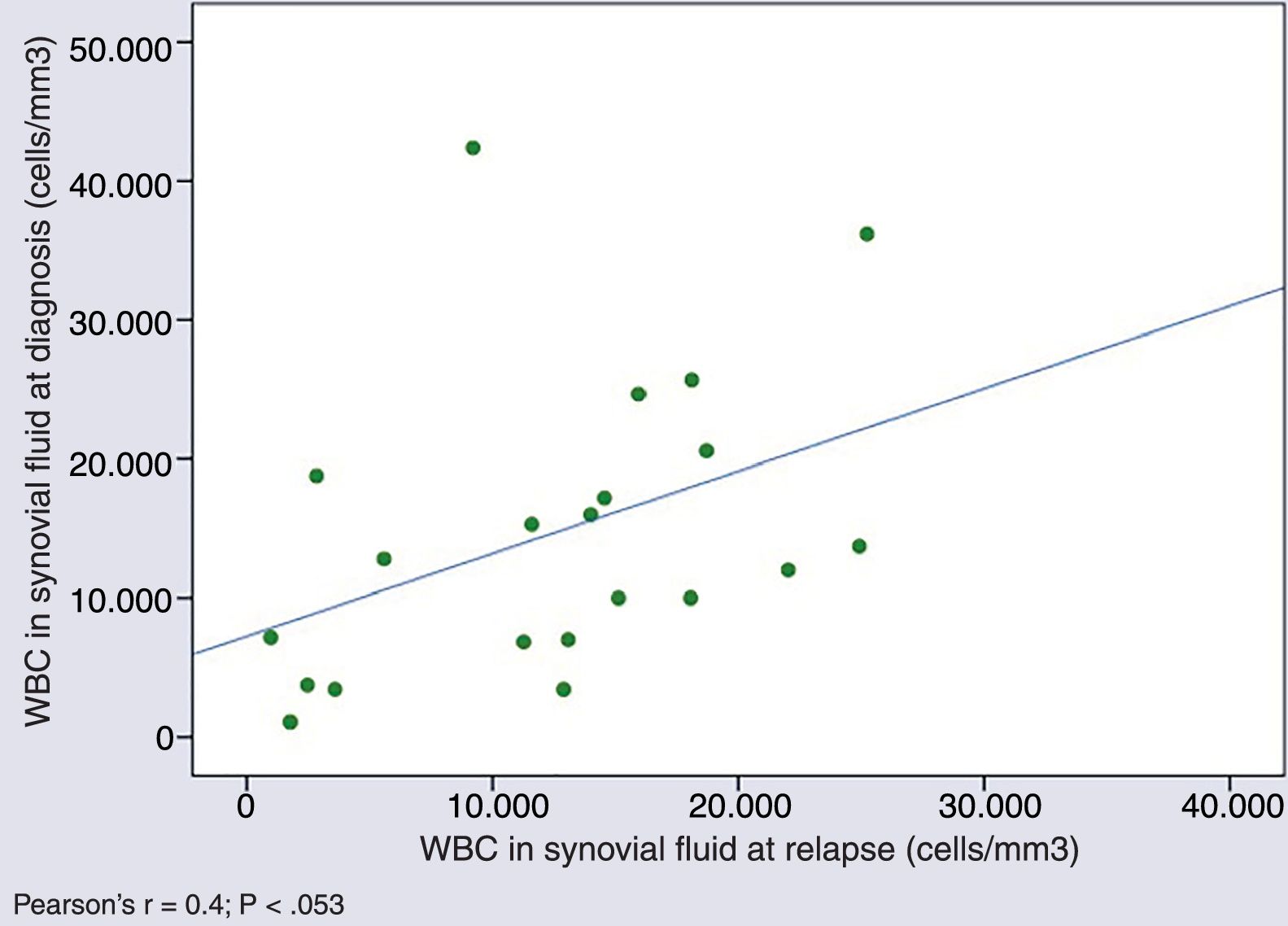
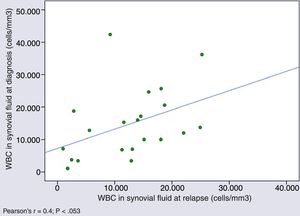
Cytological characteristics of synovial fluid over the course of diseaseWe found a tendency towards a positive correlation in the WBC counts in SF samples obtained in different episodes in patients that experienced relapses (Pearson's r, 0.4; P=.053) (Fig. 2).
In 3 out of 8 patients with WBC counts of less than 2000cells/mm3 at the time of diagnosis, an additional arthrocentesis was performed after a median of 12 months (IQR, 7–16), revealing persistence of counts of less than 2000cells persisted and predominance of MN cells in all.
Relationship between synovial fluid cell counts and disease activityLastly, we analysed the association between the level of disease activity, assessed by means of the JADAS-10, and the various SF parameters, including the WBC count, WBC subtypes, and protein concentration by means of Spearman's rho, and we found no correlation.
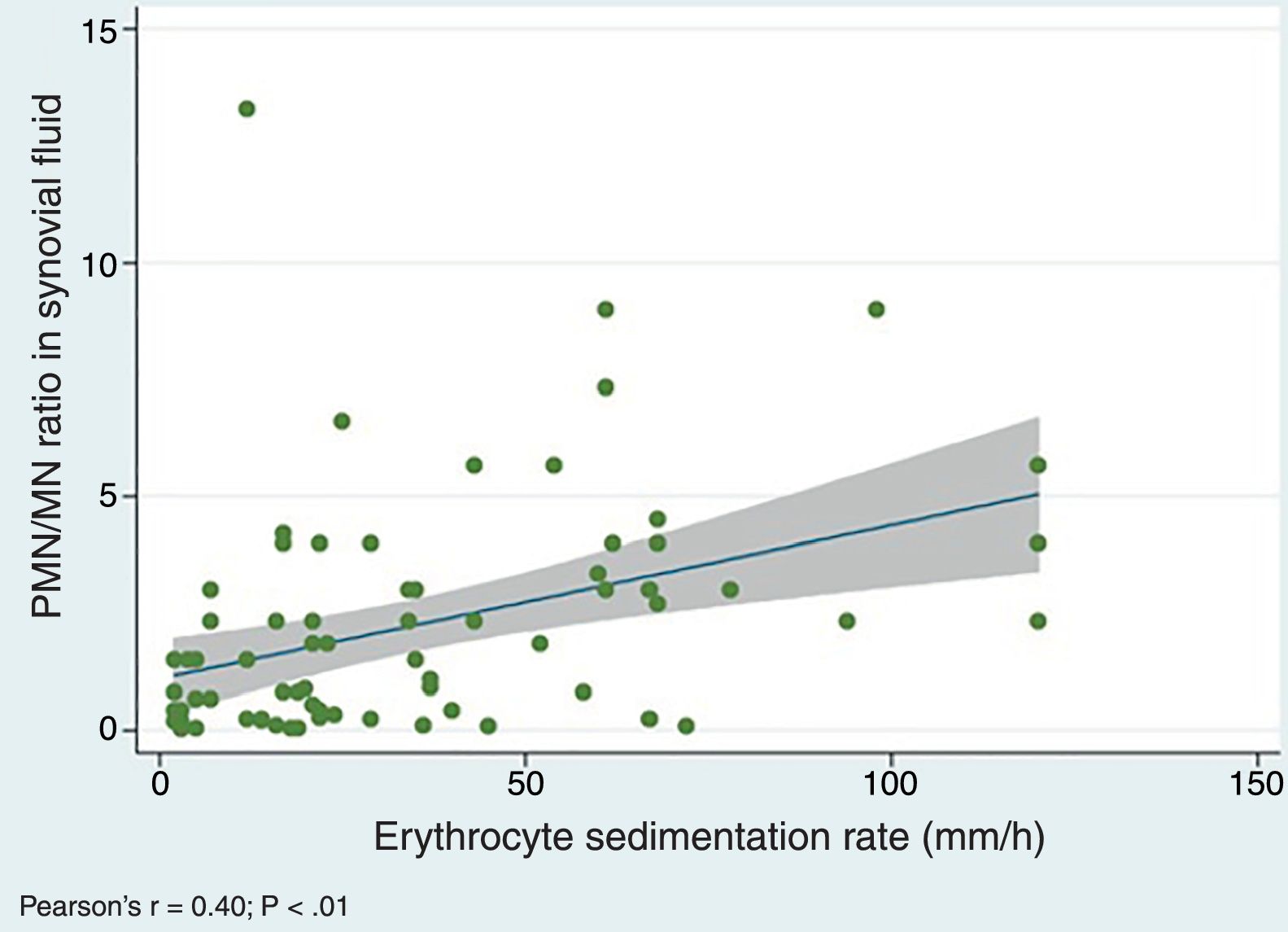
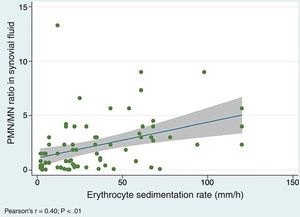
We did find a positive correlation between the PMN/MN percentage ratio in SF and the values of both the ESR (Pearson's r, 0.40; P=.0005) (Fig. 3) and the serum level of CRP (r de Pearson's r 0.25; P=.03), which suggests that the proportion of PMN in SF tends to increase with acute phase reactant values.
DiscussionSynovial fluid analysis is a fundamental tool in the investigation of arthritis, as it guides the diagnosis in a rapid and simple way. In spite of this, few studies have analysed whether clinical characteristics, such as the type of JIA or the number of involved joints, or laboratory characteristics, such as the presence of ANA or histocompatibility antigen HLA-B27, are associated with variation in the SF of patients with JIA.
The demographic characteristics of the patients in our sample were similar to those described in the literature, including the predominance of female patients in all clinical forms except systemic-onset JIA and ERA,2,12,13 the predominance of oligoarticular JIA or the higher frequency of ANA detection in patients diagnosed at younger ages.2,14,15
Although the findings of SF analysis are only pathognomonic when a microorganism is isolated in culture or crystals are detected,3 the WBC count in SF is the test used most widely to discriminate an inflammatory from a non-inflammatory condition of the joint. Shmerling et al. reported a specificity and sensitivity of 84% for a WBC count greater than 2000/mm3 for diagnosis of inflammatory arthritis.5,16 When it comes to JIA, while there are not many case series with which we can compare our results, our results are consistent with the previous literature in finding inflammatory characteristics in the SF of patients with JIA.6,17,18
In terms of the association of cell counts with the number of involved joints, we found that patients with polyarticular forms had higher WBC counts in SF compared to patients with oligoarticular forms; this difference, also reported by Punzi et al.6 and Kunnamo and Pelkonen,19 was not statistically significant in our sample.
However, our study also found some limitations in the use of cell counts in patients with JIA that clinicians should be aware of. Thus, 11 of our patients, which amounts to nearly 1 in 5, had WBC counts in SF of more than 50000 (3/11) or less than 2000/mm3 (8/11) at the time of diagnosis, so they could have been given an incorrect diagnosis of septic arthritis or non-inflammatory arthritis, respectively. The results of culture, the course of the disease and the response to treatment confirmed the diagnosis of JIA in every case. This has also been observed by other authors in patients with juvenile arthritis.17,18,20 Therefore, SF cell counts should be considered just one in several tools for the assessment of paediatric arthritis and ought to be interpreted taking into account the clinical context of each patient.
The information obtained by cell counts includes the differential count of WBC subtypes and not only the total number. The percentage of PMN cells is usually greater than 85% in cases of septic arthritis,16 while it ranges between 50% and 90% in cases of non-infectious inflammatory disease.5,6,16,20 It is generally accepted that the SF of patients with JIA is characterised by a predominance of PMN cells, as was the case in our series, although there are also authors that have reported a higher percentage of MN cells.21 When it came to the association between the number of involved joints and the differential WBC count in SF, several studies have found a higher percentage of PMN cells in polyarticular forms of disease,5,6,16,18 although our data did not corroborate this association.
Within the overall heterogeneity that characterises this disease, there is a subset of patients, those who test positive for ANA, that share specific clinical22,23 and immunological24 features to the point that some authors propose the recognition of a distinct category within JIA. These patients tend to have an early onset of disease (before age 6 years), be female, have asymmetrical joint involvement, either oligoarticular or polyarticular, and are at higher risk of developing chronic anterior uveitis.22 In addition to sharing these clinical peculiarities, these patients also exhibit a very similar cytokine profile.24
The analysis of SF characteristics based on the presence or absence of ANA in serum revealed that WBC counts in the SF of ANA-positive patients were lower compared to those of ANA-negative patients, although this difference was not statistically significant. This association has not been reported in the previous literature, so it would be very interesting to study it in larger samples if it is observed again.
When we analysed WBC counts based on the detection of HLA-B27, we found that patients who were HLA-B27-positive had clearly higher WBC counts and a higher proportion of PMN cells, although, unlike the study of Kunnamo and Pelkonen,19 these differences were not statistically significant in our case series. In addition, we found no differences in the characteristics of the SF between patients with different types of JIA, although, as occurred in our analysis of patients that tested positive for ANA or HLA-B27 antigen, these analyses could be limited by the small sample size.
Our study revealed that WBC counts in SF samples obtained in the same arthrocentesis appointment were very similar, regardless of the site of collection. Furthermore, while there is previous evidence that the ESR, the level of CRP and the number of actively involved joints decreases with treatment,25,26 we did not find decreases in the cell counts in SF during relapses.
Several authors27,28 have demonstrated that there is a strong correlation between JIA and the JADAS when the CRP level is used instead of the ESR, making it a valid tool for assessment of disease activity. In our sample, while we found that the percentage of PMN cells in SF tended to increase with the values of both acute phase reactants, we did not find differences in the different SF parameters based on the disease activity of JIA assessed by means of the JADAS.
Finally, our biochemical analyses of SF corroborated that glucose and protein concentrations are poor markers of inflammation and therefore these measurements are less informative compared to other tests performed in SF.10 Contrary to other bodily fluids such as abdominal, cerebrospinal or pleural fluid, these analyses are of limited utility when performed in SF.
There are limitations to our study. The main limitation is that it was conducted in a single centre and included a limited number of patients, with the sample size in analyses further reduced upon patient stratification.
In brief, we would like to highlight that SF analysis is a key diagnostic tool in the assessment of individuals with arthritis, although in the case of patients with JIA it has certain limitations that need to be taken into account when interpreting its results. The analysis of SF samples obtained from different joints in a single episode is not useful, as their features are very similar. Out of the SF variables analysed in our study, those that were most informative were the total and differential cell counts. These counts did not change based on disease activity.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martínez del Val E, Rodríguez Martínez A, Sánchez Becerra V, Cruz Rojo J, Enríquez Merayo E, Barral Mena E, et al. Características del líquido sinovial en pacientes con artritis idiopática juvenil. An Pediatr (Barc). 2019;91:244–250.