Despite the current trend toward less aggressive therapeutic approaches, acute haematogenous osteomyelitis (AHO) continues to be a challenge and is associated with significant morbidity worldwide. Our aim was to assess whether compliance with the current protocol was achieved in 80% of cases, to identify complications and the associated risk factors, and to analyse trends in the aetiology and management of AHO in the paediatric population.
MethodsWe conducted a longitudinal, observational, single-centre study in patients with AHO aged less than 18 years admitted to a paediatric hospital between 2008 and 2018 divided in 2 cohorts (before and after 2014). We analysed data concerning demographic and clinical characteristics and outcomes.
ResultsThe study included 71 children with AHO, 56% male, with a median age of 3 years (interquartile range, 1–11). We found a 1.8-fold increase of cases in the last 5 years. The causative agent was identified in 37% of cases: MSSA (54%), MRSA (4%), S. pyogenes (19%), K. kingae (12%), S. pneumoniae (8%), and N. meningitidis (4%). Complications were identified in 45% of patients and sequelae in 3.6%. In recent years, there was an increase in myositis (30% vs 7%; P=.02), septic arthritis (68 vs 37.2%; p=0.012) and in the proportion of patients treated for less than 4 weeks (37 vs 3.5%; p=0.012), with a similar sequelae rates. The risk factors associated with complications were age 3 or more years, C-reactive protein levels of 20mg/L or higher, time elapsed between onset and admission of 5 or more days and positive culture, although the only factor that continued to be significantly associated in the multivariate analysis was positive culture. The presence of complications was a risk factor for sequelae at 6 months.
ConclusionsOur study confirms that AHO can be aggressive. The identification of risk factors for complications is essential for management.
Aunque actualmente se tiende a un abordaje terapéutico menos agresivo, la osteomielitis hematógena aguda (OHA) sigue suponiendo un reto, con una morbilidad significativa a nivel mundial. El objetivo del estudio fue evaluar si se alcanzó una adherencia del 80% con el protocolo vigente, identificar las complicaciones y riesgos asociados y analizar las tendencias en la etiología y el manejo de la OHA en la población pediátrica.
MétodosEstudio observacional longitudinal unicéntrico en pacientes menores de 18 años con OHA ingresados en un hospital pediátrico entre 2008 y 2018 divididos en 2 cohortes (antes y después de 2014). Se analizaron datos demográficos, clínicos y concernientes a la evolución de la enfermedad.
ResultadosEl estudio incluyó a 71 niños con OHA, 56% varones, con una edad mediana de 3 años (rango intercuartílico, 1–11). Se observó una incidencia 1.8 veces mayor en los últimos 5 años. El agente causal se identificó en el 37% de los casos: SASM (54%), SARM (4%), S. pyogenes (19%), K. kingae (12%), S. pneumoniae (8%) y N. meningitidis (4%). Se identificaron complicaciones en el 45% y secuelas en 3.6% de los pacientes. En los últimos años aumentó la incidencia de miositis (30% vs 7%; p=0,02) y de artritis séptica (después de 2015, 68 vs 37.2%, p=0,012), así como la proporción de pacientes con tratamiento inferior a 4 semanas (37 vs 3.5%; p=0,012), con tasas de secuelas similares. Los factores de riesgo de complicaciones fueron la edad ≥ 3 años, nivel de Proteina C-reactiva≥20mg/L, duración de los síntomas al ingreso de 5 o más días y cultivo positivo, aunque en el análisis multivariado solo se validó el cultivo positivo. La presencia de complicaciones se identificó como factor de riesgo de secuelas a los 6 meses.
ConclusionesEl presente estudio confirma que la OHA puede ser agresiva. La identificación de los factores de riesgo es fundamental para su abordaje.
Acute haematogenous osteomyelitis (AHO) still remains a challenge, as it is associated with a significant worldwide morbidity and often requires prolonged treatment.1,2 In Europe, the incidence ranges from 2 to 13 cases per 100,000 inhabitants.2–4 The aetiology is variable, but Staphylococcus aureus is yet the most frequent causative pathogen, accounting for 36.5–70% of positive cases,1,2,5 followed by Kingella kingae in young children (14–22.2%).6,7
In Europe, empiric antibiotherapy for AHO is well established,3 but the indication for a surgical procedure remains controversial and there is a trend towards less aggressive and shorter treatments.1,8–11 However, in cases caused by S. aureus infection, especially those involving Panton-Valentine leucocidin-positive S. aureus (PVL-SA) or methicillin-resistant S. aureus (MRSA),3,12–14 treatment is longer and more aggressive when compared to cases of K. kingae infection.6,7 Furthermore, if AHO is concomitant with septic arthritis, abscesses or (pyo)myositis, prolonged treatment courses and multiple surgical interventions are often needed.1–3,13,15–17 However, few studies have identified predictors of complications, which are of outmost importance in heterogeneous series.16,18,19
In 2014, a revised protocol was introduced to manage AHO at our hospital. The objectives of our study were to identify children at higher risk for complicated AHO, analyse aetiological trends and management standard practices (to achieve 80% compliance with the current protocol) and to compare differences between S. aureus infections and infections by other pathogens.
Patient and methodsWe performed a longitudinal, observational study in children aged less than 18 years with AHO admitted to a tertiary care paediatric hospital between January 2008 and December 2018. We included patients retrospectively until 2014 and prospectively thereafter. A multidisciplinary team followed all patients from 2014. We collected data on demographic characteristics, clinical manifestations, microbiological and imaging findings, treatment and outcomes from medical charts. We excluded patients aged less than 3 months and patients with a history of surgery or open trauma at the site of infection.
The diagnosis of AHO and septic arthritis was based on classical clinical findings (inability to bear weight, limited range of movement [ROM], pain, local inflammatory signs, fever) for less than 14 days and compatible imaging findings, with or without microbial isolation.1,2 Good clinical response on follow-up further indicated septic arthritis. Complications included abscesses, (pyo)myositis, deep venous thrombosis (DVT), disseminated infection, pathologic fractures, joint dislocation, avascular necrosis or chronic osteomyelitis. Residual pain, limping, ROM limitation, stiffness, angular deformity or limb length discrepancy (LLD) caused by AHO and lasting for more than 6 months were considered sequelae.1,16
The recommended first-line antibiotics were flucloxacillin (150−200mg/kg/day given every 6h) or cefuroxime (150mg/kg/day given every 8h) for children aged 3 years or younger. A switch to oral therapy was considered after a minimum of 3 days if the patient was afebrile, improving clinically and had a 50% decrease in the C-reactive protein (CRP) level (or CRP≤20mg/L).3,10
Bacterial identification and antimicrobial susceptibility were performed at the hospital microbiology laboratory. Synovial fluid samples for culture were inoculated into blood culture bottles. After 2014, when available, joint fluid and bone samples were tested for Kingella kingae with specific real-time polymerase chain reaction (PCR), in children aged 4 years or younger.
Statistical analysisValues were expressed as percentages for qualitative variables or as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables. Continuous variables were compared with the Student t-test, Mann–Whitney U test or Kruskal–Wallis test and categorical variables with the chi square test or Fisher exact test. We used the Pearson correlation coefficient to assess the association between 2 continuous variables. A multivariate logistic regression analysis was performed to identify potential risk factors associated with complications and sequelae. SPSS Statistics® version 24 (IBM Corp, New York, USA) was used to perform data analysis. We considered p-values of less than 0.05 statistically significant. Data was collected and handled in accordance with the General Regulation on Data Protection (EU) 2016/679 of 27 April 2016, and the study was approved by the local ethics committee.
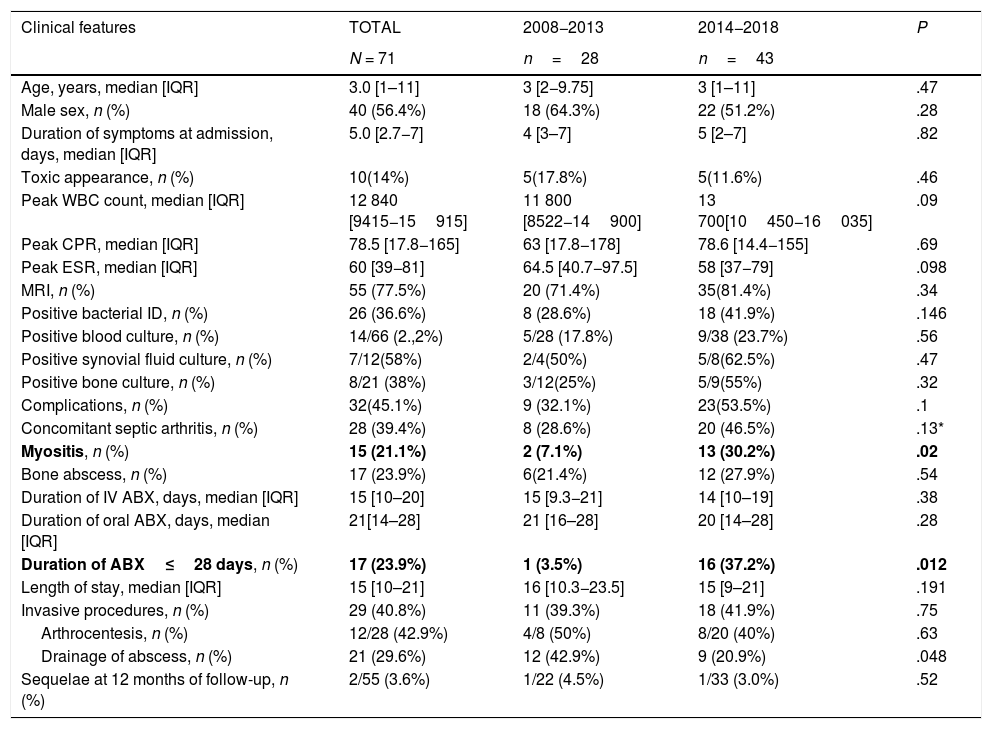
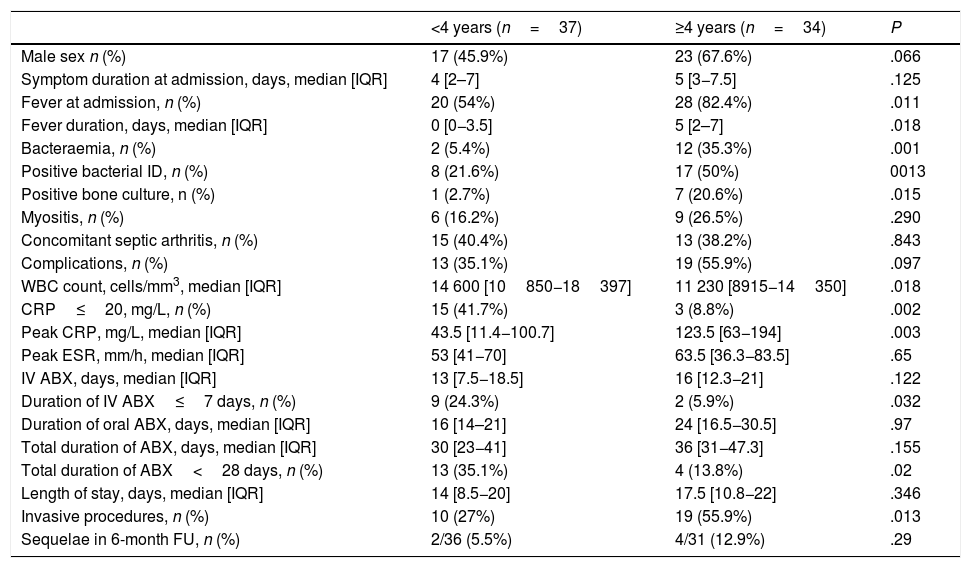
ResultsOverall study populationWe identified a total of 71 cases of AHO, corresponding to a mean annual incidence of 11.4 cases per 100,000 children (range, 3–10 cases/year), of which 42% were diagnosed in the winter months. We observed a 1.8-fold increase in incidence in the last 5 years. Table 1 summarizes patient clinical features. Twelve children (16.9%) reported previous skin lesions (3 had chickenpox), and 8had chronic diseases (7 sickle cell disease, 1 chronic kidney disease). Table 2 compares the clinical features of children aged less than 4 years and 4 years and older.
Demographic, clinical and laboratory characteristics of the total sample.
| Clinical features | TOTAL | 2008−2013 | 2014−2018 | P |
|---|---|---|---|---|
| N = 71 | n=28 | n=43 | ||
| Age, years, median [IQR] | 3.0 [1–11] | 3 [2−9.75] | 3 [1–11] | .47 |
| Male sex, n (%) | 40 (56.4%) | 18 (64.3%) | 22 (51.2%) | .28 |
| Duration of symptoms at admission, days, median [IQR] | 5.0 [2.7−7] | 4 [3–7] | 5 [2–7] | .82 |
| Toxic appearance, n (%) | 10(14%) | 5(17.8%) | 5(11.6%) | .46 |
| Peak WBC count, median [IQR] | 12 840 [9415−15915] | 11 800 [8522−14900] | 13 700[10450−16035] | .09 |
| Peak CPR, median [IQR] | 78.5 [17.8−165] | 63 [17.8−178] | 78.6 [14.4−155] | .69 |
| Peak ESR, median [IQR] | 60 [39−81] | 64.5 [40.7−97.5] | 58 [37−79] | .098 |
| MRI, n (%) | 55 (77.5%) | 20 (71.4%) | 35(81.4%) | .34 |
| Positive bacterial ID, n (%) | 26 (36.6%) | 8 (28.6%) | 18 (41.9%) | .146 |
| Positive blood culture, n (%) | 14/66 (2.,2%) | 5/28 (17.8%) | 9/38 (23.7%) | .56 |
| Positive synovial fluid culture, n (%) | 7/12(58%) | 2/4(50%) | 5/8(62.5%) | .47 |
| Positive bone culture, n (%) | 8/21 (38%) | 3/12(25%) | 5/9(55%) | .32 |
| Complications, n (%) | 32(45.1%) | 9 (32.1%) | 23(53.5%) | .1 |
| Concomitant septic arthritis, n (%) | 28 (39.4%) | 8 (28.6%) | 20 (46.5%) | .13* |
| Myositis, n (%) | 15 (21.1%) | 2 (7.1%) | 13 (30.2%) | .02 |
| Bone abscess, n (%) | 17 (23.9%) | 6(21.4%) | 12 (27.9%) | .54 |
| Duration of IV ABX, days, median [IQR] | 15 [10–20] | 15 [9.3−21] | 14 [10–19] | .38 |
| Duration of oral ABX, days, median [IQR] | 21[14–28] | 21 [16–28] | 20 [14–28] | .28 |
| Duration of ABX≤28 days, n (%) | 17 (23.9%) | 1 (3.5%) | 16 (37.2%) | .012 |
| Length of stay, median [IQR] | 15 [10–21] | 16 [10.3−23.5] | 15 [9–21] | .191 |
| Invasive procedures, n (%) | 29 (40.8%) | 11 (39.3%) | 18 (41.9%) | .75 |
| Arthrocentesis, n (%) | 12/28 (42.9%) | 4/8 (50%) | 8/20 (40%) | .63 |
| Drainage of abscess, n (%) | 21 (29.6%) | 12 (42.9%) | 9 (20.9%) | .048 |
| Sequelae at 12 months of follow-up, n (%) | 2/55 (3.6%) | 1/22 (4.5%) | 1/33 (3.0%) | .52 |
ABX, antibiotherapy; CPR, C-reactive protein; ESR, erythrocyte sedimentation rate; ID, identification; IQR, interquartile range; WBC, white blood cell.
Distribution of clinical features by age group.
| <4 years (n=37) | ≥4 years (n=34) | P | |
|---|---|---|---|
| Male sex n (%) | 17 (45.9%) | 23 (67.6%) | .066 |
| Symptom duration at admission, days, median [IQR] | 4 [2–7] | 5 [3−7.5] | .125 |
| Fever at admission, n (%) | 20 (54%) | 28 (82.4%) | .011 |
| Fever duration, days, median [IQR] | 0 [0−3.5] | 5 [2–7] | .018 |
| Bacteraemia, n (%) | 2 (5.4%) | 12 (35.3%) | .001 |
| Positive bacterial ID, n (%) | 8 (21.6%) | 17 (50%) | 0013 |
| Positive bone culture, n (%) | 1 (2.7%) | 7 (20.6%) | .015 |
| Myositis, n (%) | 6 (16.2%) | 9 (26.5%) | .290 |
| Concomitant septic arthritis, n (%) | 15 (40.4%) | 13 (38.2%) | .843 |
| Complications, n (%) | 13 (35.1%) | 19 (55.9%) | .097 |
| WBC count, cells/mm3, median [IQR] | 14 600 [10850−18397] | 11 230 [8915−14350] | .018 |
| CRP≤20, mg/L, n (%) | 15 (41.7%) | 3 (8.8%) | .002 |
| Peak CRP, mg/L, median [IQR] | 43.5 [11.4−100.7] | 123.5 [63−194] | .003 |
| Peak ESR, mm/h, median [IQR] | 53 [41−70] | 63.5 [36.3−83.5] | .65 |
| IV ABX, days, median [IQR] | 13 [7.5−18.5] | 16 [12.3−21] | .122 |
| Duration of IV ABX≤7 days, n (%) | 9 (24.3%) | 2 (5.9%) | .032 |
| Duration of oral ABX, days, median [IQR] | 16 [14–21] | 24 [16.5−30.5] | .97 |
| Total duration of ABX, days, median [IQR] | 30 [23−41] | 36 [31−47.3] | .155 |
| Total duration of ABX<28 days, n (%) | 13 (35.1%) | 4 (13.8%) | .02 |
| Length of stay, days, median [IQR] | 14 [8.5−20] | 17.5 [10.8−22] | .346 |
| Invasive procedures, n (%) | 10 (27%) | 19 (55.9%) | .013 |
| Sequelae in 6-month FU, n (%) | 2/36 (5.5%) | 4/31 (12.9%) | .29 |
ABX, antibiotherapy; CPR, C-reactive protein; ESR, erythrocyte sedimentation rate; FU, follow-up; ID, identification; IQR, interquartile range; WBC, white blood cell.
The most frequent clinical findings were pain (95.8%), ROM limitation (85.9%), fever (tympanic temperature ≥ 38.2°C; 67.6%), local inflammatory signs (91.5%), irritability (7%) and toxic appearance (14.1%). The bones involved most frequently were the femur (22.5%), tibia (19.7%), humerus (14.1%), iliac bone (11.3%) and the tarsal bones (11.3%). The most frequently affected joints were the hip (12.7%), knee (5.6%), elbow (5.6%), ankle (5.6%) and shoulder (4.2%). Six (8.5%) patients had multifocal involvement. A plain radiograph was performed in 93% of the patients and was normal in 66.7%. Magnetic resonance imaging (MRI) was performed in 77.5% patients with a high diagnostic yield (98%). The use of MRI did not increase significantly with the implementation of the protocol.
MicrobiologyA causative microbial pathogen was identified in 36.6% of cases either by positive culture (24/65) or by molecular amplification (2/7). Blood cultures were performed in 98.5% of patients, and 21.2% were positive. Real-time PCR for K. kingae was only performed in 5 patients: 3 in bone (all negative), 1 in synovial fluid (positive) and 2 in oropharyngeal swabs (1 positive). In addition, PCR for bacterial 16s RNA gene was only done in 2 patients and was negative in both. Six patients had persistent positive cultures for S. aureus 5–22 days after receiving appropriate antibiotic treatment (isolated from blood [3] or bone abscesses [3])–. These patients had concomitant bone abscesses (4), myositis (3), fasciitis (2), sepsis/disseminated disease (2) and DVT (1). All needed invasive procedures and at least 14 days of IV antibiotic treatment.
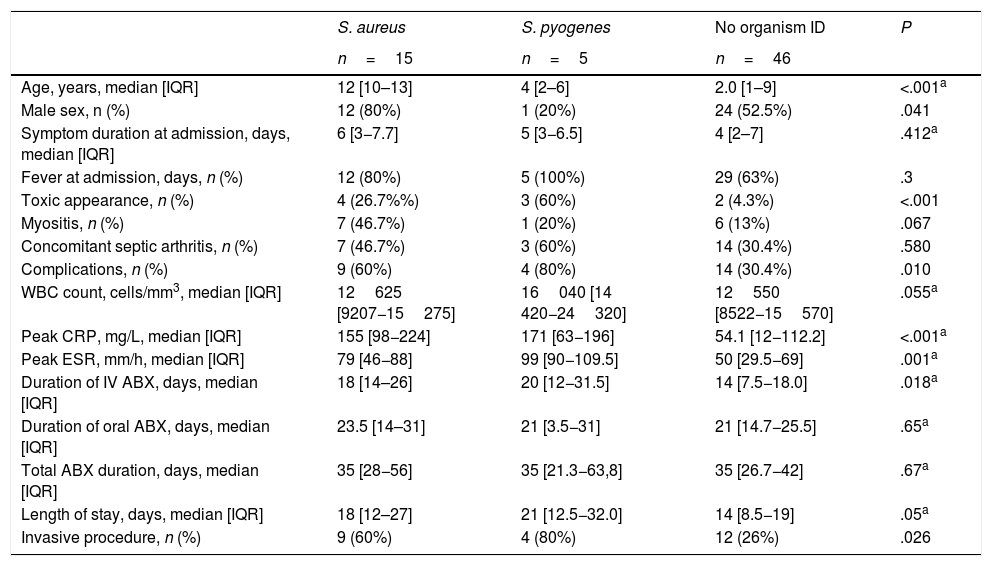
The most commonly identified bacteria were S. aureus (15/26; 57.7%), Streptococcus pyogenes (5/26; 19.2%), K. kingae (3/26; 11.5%), Streptococcus pneumoniae (2/26; 7.7%) and Neisseria meningitidis (1/26; 3.8%). Out of all S. aureus isolates, 93.3% were methicillin-susceptible S. aureus (MSSA). Table 3 compares the clinical features of cases with isolation of S. aureus, S. pyogenes and without isolation. Cases without microbiological identification were more common in children younger than 4 years, who had shorter fever duration (3.1 vs 5.3 days; P=.05), without toxic appearance (93.5% vs 72%, P=.013), but with CRP levels of 20mg/L or less (35.6% vs 8%; P=.011) and a duration of IV antibiotherapy of less than 7 days (23.9% vs 0%, P=.008).
Clinical features by infectious agent.
| S. aureus | S. pyogenes | No organism ID | P | |
|---|---|---|---|---|
| n=15 | n=5 | n=46 | ||
| Age, years, median [IQR] | 12 [10–13] | 4 [2–6] | 2.0 [1–9] | <.001a |
| Male sex, n (%) | 12 (80%) | 1 (20%) | 24 (52.5%) | .041 |
| Symptom duration at admission, days, median [IQR] | 6 [3−7.7] | 5 [3−6.5] | 4 [2–7] | .412a |
| Fever at admission, days, n (%) | 12 (80%) | 5 (100%) | 29 (63%) | .3 |
| Toxic appearance, n (%) | 4 (26.7%%) | 3 (60%) | 2 (4.3%) | <.001 |
| Myositis, n (%) | 7 (46.7%) | 1 (20%) | 6 (13%) | .067 |
| Concomitant septic arthritis, n (%) | 7 (46.7%) | 3 (60%) | 14 (30.4%) | .580 |
| Complications, n (%) | 9 (60%) | 4 (80%) | 14 (30.4%) | .010 |
| WBC count, cells/mm3, median [IQR] | 12625 [9207−15275] | 16040 [14 420−24320] | 12550 [8522−15570] | .055a |
| Peak CRP, mg/L, median [IQR] | 155 [98−224] | 171 [63−196] | 54.1 [12−112.2] | <.001a |
| Peak ESR, mm/h, median [IQR] | 79 [46−88] | 99 [90−109.5] | 50 [29.5−69] | .001a |
| Duration of IV ABX, days, median [IQR] | 18 [14–26] | 20 [12−31.5] | 14 [7.5−18.0] | .018a |
| Duration of oral ABX, days, median [IQR] | 23.5 [14–31] | 21 [3.5−31] | 21 [14.7−25.5] | .65a |
| Total ABX duration, days, median [IQR] | 35 [28−56] | 35 [21.3−63,8] | 35 [26.7−42] | .67a |
| Length of stay, days, median [IQR] | 18 [12–27] | 21 [12.5−32.0] | 14 [8.5−19] | .05a |
| Invasive procedure, n (%) | 9 (60%) | 4 (80%) | 12 (26%) | .026 |
ABX, antibiotherapy; CPR, C-reactive protein; ESR, erythrocyte sedimentation rate; FU, follow-up; ID, identification; IQR, interquartile range; WBC, white blood cell.
Children with S. aureus infection had a higher proportion of patients aged more than 5 years (86.7% vs 35.7%; P<.001), with CRP levels of 20mg/L or greater (100% vs 67.2%; P=.010), had more frequently myositis (46.7% vs 14.3%; P=.0006), and a longer duration of IV antibiotherapy (18 vs 14 days; P=.04). Only one of these patients, who had AHO caused by MRSA, exhibited sequelae at 6 months. This patient had severe multisystem, multifocal infection by LPV-negative MRSA (this was the only strain tested for LPV) and was treated with vancomycin plus rifampicin, followed by linezolid due to persistent bacteraemia. At 24 months’ follow-up, the restricted hip mobility persisted. Group A streptococcus (GAS) infections were also severe, manifesting with fever, toxic appearance (60%), elevation of inflammatory markers and complications such as myositis (in 1 patient), subperiosteal/intraosseous abscesses (in 2) and fasciitis/compartment syndrome (in 1). However, none of these patients had sequelae at 12 months of follow-up. The only GAS isolate submitted to molecular typing was an emm4 strain.
All 3 patients with K. kingae infection were aged less than 2 years and presented without fever and with low CRP levels. Kingella kingae was identified by molecular amplification in 2 cases and by bone culture in 1. One of these patients had hip subluxation, although the abnormalities had resolved by 12 months of follow-up.
Patients with bacteraemia were more likely to have S. aureus infection (75% vs 10%; P<.000), longer duration of fever (6 vs 3.4 days; P=.053) and a CRP level of 20mg/L or greater (100% vs 70%; P=.019).
Patients with concomitant septic arthritis were more likely to have received the diagnosis after 2015 (68% vs 37.2%; P=.012), have an elevated ESR (68.5% vs 50%; P=.023), have an identified pathogen (50% vs 25.5%; P=.035) and myositis (35.7% vs 11.6%; P=.015). Patients with myositis were more likely to have S. aureus infection (46.7% vs 14.3%; P=.006), septic arthritis, a white blood cell count greater than 15000 cells/mm3 (53.3% vs 26.8%; P=.05) and CRP levels of 20mg/L or greater (93% vs 67.8%; P=.05), and they received more than 7 days of IV antibiotherapy (100% vs 26.8%; P=.02), without differences in the frequency of invasive treatment.
ManagementPerformance of arthrocentesis was similar in both study periods, with 12/28 (42.9%) of patients with septic arthritis undergoing the procedure. In the remaining patients (16/28) there was not enough fluid for drainage, but 4 underwent bone aspiration. Performance of bone biopsies and abscess drainage was less frequent after 2014. In 7 patients (6 after 2014), abscesses were not drained, and only 2 patients had a bone biopsy sample collected for diagnosis, both before 2014.
In our sample, 11/71 patients (15.5%) required more than one invasive intervention: 72.7% were aged 4 years or older, all had positive cultures, 11 had concomitant septic arthritis, 5 had abscesses and 4 had myositis.
Complications and sequelaeComplications developed in 45.1% patients, and included intraosseous or subperiosteal abscesses (23.9%), myositis (21.1%), avascular necrosis (2.8%), subluxation (1.4%), pathological fracture (1.4%), fasciitis (4.2%), sepsis (1.4%) and DVP (1.4%). Four patients (5.6%) required intensive care support and 3 had disseminated disease (1.4%). At discharge, 16.9% had residual symptoms, chiefly limited ROM (14.1%) and stiffness (2.8%).
Four (5.6%) patients were lost to follow-up after the initial treatment but all had no symptoms at discharge. At 6 months of follow-up, 6/67 patients (9%) had sequelae: limited ROM (n=2), LLD (n=1), stiffness (n=1), angular deformity (n=1) and residual pain (n=1). At 12 months of follow-up, 2/55 patients (3.6%) had sequelae: ROM limitation (n=1, corresponding to the patient with MRSA) and joint stiffness (n=1).
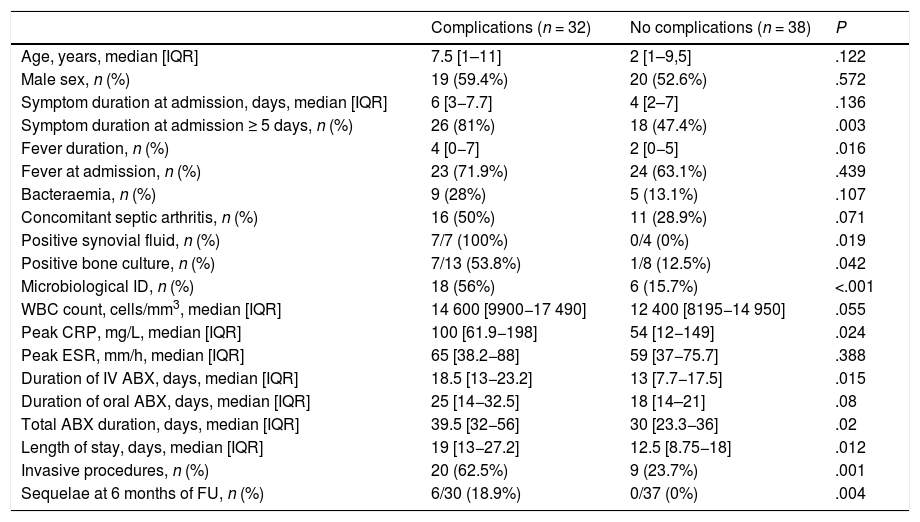
Predictors of poor outcomeThe length of stay was associated with the duration of fever (r=0.3; P=.06), the levels of inflammatory markers at admission, such as CRP (r=0.53; P<.001) or the ESR (r=0.41; P<.001) and the number of surgical interventions (r=0.5; P<.001). Risk factors for complications (Table 4) were age 3 or more years (OR, 3.37 [1.25–9.08]), time from onset of symptoms to diagnosis of 5 days or longer (OR, 4.8 [1.61–14.3]), CRP level of 20mg/L or greater (OR, 4.26 [1.23–14.7]) and microbiological isolation (OR, 6.8 [2.2–20.9]. In the multivariate analysis, the only factor that continued to be associated with the development of complications was microbiological isolation. The presence of sequelae at 6 months was also associated with the presence of complications (OR, 4.4 [1.66–11.6]).
Comparison of patients with complicated vs uncomplicated infections.
| Complications (n = 32) | No complications (n = 38) | P | |
|---|---|---|---|
| Age, years, median [IQR] | 7.5 [1–11] | 2 [1–9,5] | .122 |
| Male sex, n (%) | 19 (59.4%) | 20 (52.6%) | .572 |
| Symptom duration at admission, days, median [IQR] | 6 [3−7.7] | 4 [2–7] | .136 |
| Symptom duration at admission ≥ 5 days, n (%) | 26 (81%) | 18 (47.4%) | .003 |
| Fever duration, n (%) | 4 [0−7] | 2 [0−5] | .016 |
| Fever at admission, n (%) | 23 (71.9%) | 24 (63.1%) | .439 |
| Bacteraemia, n (%) | 9 (28%) | 5 (13.1%) | .107 |
| Concomitant septic arthritis, n (%) | 16 (50%) | 11 (28.9%) | .071 |
| Positive synovial fluid, n (%) | 7/7 (100%) | 0/4 (0%) | .019 |
| Positive bone culture, n (%) | 7/13 (53.8%) | 1/8 (12.5%) | .042 |
| Microbiological ID, n (%) | 18 (56%) | 6 (15.7%) | <.001 |
| WBC count, cells/mm3, median [IQR] | 14 600 [9900−17 490] | 12 400 [8195−14 950] | .055 |
| Peak CRP, mg/L, median [IQR] | 100 [61.9−198] | 54 [12−149] | .024 |
| Peak ESR, mm/h, median [IQR] | 65 [38.2−88] | 59 [37−75.7] | .388 |
| Duration of IV ABX, days, median [IQR] | 18.5 [13−23.2] | 13 [7.7−17.5] | .015 |
| Duration of oral ABX, days, median [IQR] | 25 [14−32.5] | 18 [14–21] | .08 |
| Total ABX duration, days, median [IQR] | 39.5 [32−56] | 30 [23.3−36] | .02 |
| Length of stay, days, median [IQR] | 19 [13−27.2] | 12.5 [8.75−18] | .012 |
| Invasive procedures, n (%) | 20 (62.5%) | 9 (23.7%) | .001 |
| Sequelae at 6 months of FU, n (%) | 6/30 (18.9%) | 0/37 (0%) | .004 |
ABX, antibiotherapy; CPR, C-reactive protein; ESR, erythrocyte sedimentation rate; FU, follow-up; ID, identification; IQR, interquartile range; WBC, white blood cell.
Although our study was conducted in a single centre, it included one of the largest cohorts of patients with AHO to describe trends in the aetiology and management of AHO in Lisbon and Southern Portugal. Our findings show that (1) MSSA continues to be the most common causative agent of AHO, with no variation between years; (2) K. kingae is still not a frequent detectable cause of AHO in these patients, still molecular testing continues not to be performed routinely; (3) the management of AHO in our area continues to be traditional, with prolonged courses of antibiotherapy, longer lengths of stay and a higher frequency of surgical intervention, although this trend has been decreasing in recent years; (4) there is an unexpectedly high frequency of concomitant septic arthritis and complications, mostly myositis, which may explain some of these results. The increased accessibility and precision of MRI in recent years may also partly account for some of our findings. However, our study did not find an increase in the use of MRI over time.
The incidence of AHO has increased in recent years.2 Gafur et al. report a 2.8-fold increase in the last 20 years,4 while Samara et al. describe a 79% increase in the mean annual incidence,7 although only in young children. Similarly, we found a 1.8-fold increase in the past 5 years, but without changes in age distribution. A growing awareness of AHO, prospective enrolment after 2014 and improvements in imaging combined with an actual incidence rise may explain this trend. The use of molecular detection methods, which was minimal in our study, cannot account for this increase.
In our study, a pathogen was identified in only 36.6% of patients, which was consistent with the findings of Filleron et al. in France (40.7%)20 or Calvo et al in Spain (33%).16 Yet, it is much lower than the 62.7% reported by Samara et al in Geneva,7 where they routinely used molecular methods for detection of K. kingae, or the 76% reported by McNeil et al in the United States.21 In addition, the proportion of positive blood cultures in our study was only 21%, lower compared to the previous literature,1,2,10 although it was similar to the proportion reported by Samara et al.7 In our sample, infections without microbiological identification were more common in children aged less than 4 years, with CRP levels of less than 20mg/L and fewer complications. It is very likely that K. kingae caused a fair proportion of these infections in young children. In our study, only 2 of the 5 children tested for K. kingae by PCR had positive results, with detection in a synovial fluid sample in one and in a throat swab sample in the other. Synovial fluid samples were collected in 7 other cases, but the small amount of fluid precluded the use of this assay. Although diagnosis based on a positive throat swab is questionable given the prevalence of oropharyngeal colonization by K. kingae in young children (8%–12%), the throat swab seems to offer a good sensitivity (90.5%) for diagnosis of K. kingae in osteoarticular infections.7,22 The routine use of molecular techniques would increase the reported incidence of K. kingae infection,7,23 especially since the incidence of septic arthritis is increasing. In any case, bone aspiration was less frequent after 2014, which was probably associated with a decrease in microbiological identification.
Staphylococcus aureus was the bacterium isolated most frequently (57.7%), as expected based on its involvement in 36.5% to 63% of cases described in the literature.1,10,13,14 In our study, most cases involved MSSA (93.3%) and MRSA was still uncommon (6.6%), which was similar to the findings in other European countries (7.8%)12 and much lower compared to the United States (18.5%–70%).13,17,24 In a previous study conducted in our hospital between 2005 and 2008, community-acquired S. aureus infections were PVL-positive in 23% of cases, with isolation of MRSA in 4.3%.25 Although MRSA infections are associated with complications and poorer outcomes, MSSA can be equally aggressive, independently of PVL production or the USA300 genotype.12–14,17 Most studies report a higher frequency of fever, a higher mean CRP value, more complications, longer antibiotic courses and longer lengths of stay in patients with S. aureus infections.13,14,17,24 Concomitant septic arthritis, subperiosteal abscess, pyomyositis and DVT are common complications of these infections.13,14,17 In our series, although we only tested for LPV in 1 case with a negative result, S. aureus infections were also associated with higher elevation of inflammatory markers, longer duration of treatment and length of stay and a higher incidence of complications (60%).
The incidence of S. pneumoniae (8%) and GAS infections remained stable in our series (19%). However, in Europe, especially in the United Kingdom, there is evidence of an increased incidence of infection by invasive GAS, specially emm1 strains.26 In our study, the only GAS strain available for typing was an emm4, which is not among the most prevalent emm types involved in invasive infections in Portugal (emm1, emm89 and emm3).27 However, this was consistent with the findings of a study conducted in Spain, in which the most frequent emm types involved in osteoarticular infections in children were emm1, emm12 and emm4.28 In our cohort, although the incidence of GAS infections did not increase, they were severe and required not only prolonged IV treatment longer hospital stays, but also multiple surgical interventions.
In our study, 39.4% of patients had concomitant septic arthritis, with an increased frequency (54.3%) since 2015. This is comparable to the 35.2% reported by McNeil et al.21 or the 39.8% reported by Schallert et al.29 but higher compared to the 12% reported in the Spanish cohort.16 Despite the high sensitivity of MRI for both AHO and septic arthritis, specific virulent factors may account for this difference.3,13,14,29 In our series, there was a clear increase in AHO with concomitant septic arthritis after 2015, which was more frequent in children with a positive culture, fever at admission and greater elevation of inflammatory markers and was associated with longer treatment duration, as observed in other studies.17,18,29
The incidence of myositis is increasing, and it has been suggested that this is related to the increase in complicated MRSA and PVL-positive infections. In 2006, Bocchini et al. reported than PVL-positive S. aureus isolates were significantly associated with a higher probability of concomitant myositis and more severe disease.13 Furthermore, Dohin et al. considered myositis as a musculoskeletal complication.14 In our study, myositis was associated with greater elevation of inflammatory markers, S. aureus infection, septic arthritis and prolonged IV treatment, suggesting an intense local inflammatory response that would contribute to disease severity.
We found adherence with the hospital treatment protocol in 80% of cases, although the duration of antibiotherapy was too long: although it had decreased modestly in recent years, it was still far from the short course of IV antibiotherapy proposed by Peltola and Pääkkönen.10 On the other hand, in their case series only 3.8% of patients had hip involvement, compared to 12.7% in our sample, and the authors did not report on complications, but in our sample 45.1% of patients experienced complications, more frequently after 2015 (54.2%). These differences may partially explain the longer duration of treatment, along with the more traditional approach used in our hospital.3,17,19 Indeed, patients with complicated high-risk osteoarticular infections or with slow clinical improvement may require longer courses of IV and oral antibiotherapy.3,19
According to the European guidelines, surgery should be avoided in uncomplicated AHO.3 In agreement with this, Peltola and Pääkkönen mention that up to 90% of patients with early AHO can be treated conservatively.10 However, in a case series previously published by these authors, only 24% of children with AHO did not require surgery.31 Calvo et al. reported surgical intervention in only 20% of patients with AHO, with most surgeries (46%) performed on account of complications, which only happened in 13.4% of patients.16 In our study, 40.8% of patients underwent invasive procedures, which were more frequent in patients with complicated AHO (62.5%), which was consistent with previous studies.11,21,24 Indeed, abscess drainage should be considered in cases in which fever, symptoms, marked elevation of CRP or bacteraemia persist.3 Our study found a less aggressive approach to the management of AHO in recent years, with a decreased frequency of bone aspiration and abscess drainage despite a similar frequency of abscesses.
Sequelae occur in 1.1%–4.5% of cases and are associated with strain virulence (more frequent in MRSA infections, 4.7%–8.6%),17,24 disease severity, delayed treatment and concomitant hip or shoulder infection.3,21,30 In our study, 3.6% of the patients had poor long-term outcomes, all minor and associated with previous complications (OR, 4.4). It is likely that strain differences and host susceptibility explain small variations in outcomes.
Identifying children at risk of complications is essential.16,19,31 In our study, the main predictors of complicated AHO were age 3 years or greater, time elapsed from onset to diagnosis of 5 or more days, CRP levels of 20mg/L or greater and pathogen isolation. Furthermore, microbiological isolation was the only factor that remained significant in the logistic regression analysis. The presence of sequelae at 6 months of follow-up was associated with complications. However, we were unable to identify clinical and laboratory predictors of sequelae of AHO due to the small sample size. Other limitations of the study include the partial retrospective design and it being conducted in a single centre.
AHO still is a major health problem. The most frequent aetiological agent continues to be MSSA, which causes more severe infections. Microbiological diagnosis in our area still needs to improve, mainly through the use of molecular testing. Duration of treatment should be reduced through the use of validated scores to identify patients with a lower risk of complications.
Previous presentation: study presented at the 19th Annual Meeting of the Portuguese Pediatric Society.
Please cite this article as: Gouveia C, Branco J, Norte S, Arcangelo J, Alves P, Pinto M, et al. Osteomielitis hematógena aguda en Lisboa: asociación con miositis y artritis inesperadamente alta. An Pediatr (Barc). 2022;96:106–114.