Neopterin and biopterin are subproducts of redox reactions, which act as cofactor of enzymes responsible for nitric oxide production. We hypothesized that plasma neopterin suffers different evolution during the first days of a critically ill child.
MethodsSingle-center prospective observational study in patients 7 days to 14 years admitted to our PICU and that met SIRS criteria. Neopterin and biopterin levels as well as other acute phase reactants were collected at admission and at 24h.
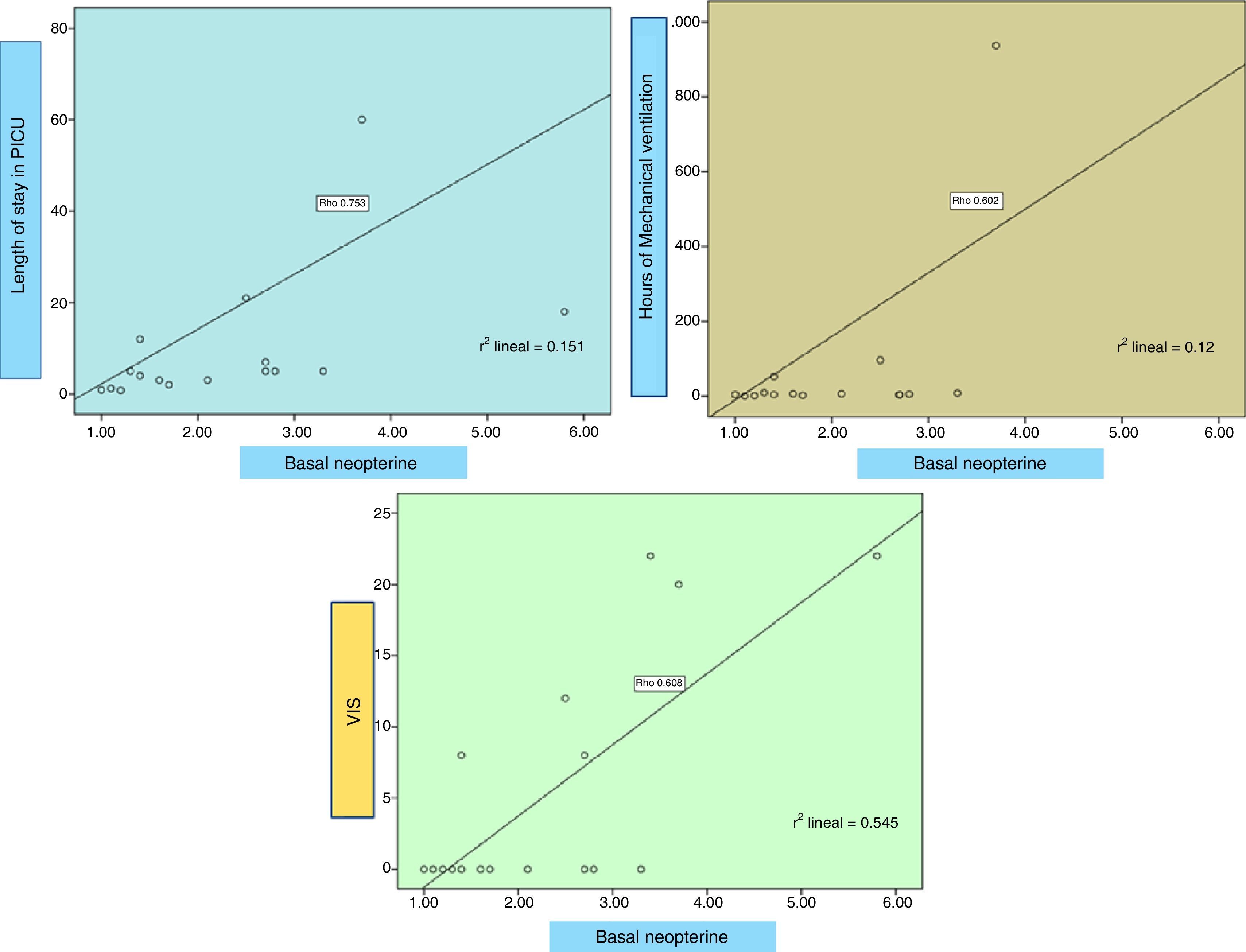
ResultsA total of 28 patients were included, 78.9% male, median age 5.04 years (interquartile range [IQR] 1.47–10.26), PRISM II 2.0% (IQR 1.1–5.0). Mechanical ventilation (MV) in 90% of patients, median duration of 6.0h (IQR 3.7–102.0); median PICU length of stay 5.0 days (IQR 2.7–18.7), maximum VIS through 0 (IQR 0–14). Baseline neopterin level 2.3±1.2nmol/l and at 24h 2.3±1.4nmol/l. Baseline biopterin is 1.3±0.5nmol/l and 1.4±0.4nmol/l at 24h. Neopterin levels significantly higher in patients with PICU length of stay >6 days (p=.02), patients who needed MV >24h (p=.023) and those who developed complications (p=.05). Neopterin correlates directly and statistically significant with the duration of MV (rho=.6, p=.011), PICU length of stay (rho=.75, p<.0001) and VIS (rho=.73, p=.001). Additionally, biopterin directly correlates with the PRISM (rho=.61, p=.008).
DiscussionThere is a higher neopterin level when longer PICU stay, higher VIS score, longer MV and occurrence of complications, pointing at the involvement of an activation of the cellular immune system.
La neopterina y biopterina, subproductos de reacciones redox, son cofactores en la producción de óxido nítrico. Hipótesis: La neopterina y biopterina plasmáticas sufren evolución diferente durante los primeros días de una enfermedad crítica en pediatría.
MétodosEstudio prospectivo observacional monocéntrico en pacientes de 7 días-14 años ingresados en UCIP con criterios de SRIS. Se recogieron, al ingreso y a las 24h, los niveles de neopterina y biopterina, otros reactantes de fase aguda y datos clínicos.
ResultadosSe analizó a 28 pacientes, el 78,9% varones, de 5,04 años (RIQ 1,47–10,26), con PRISM II 2,0% (RIQ 1,1–5,0), ventilación mecánica (VM) en 90% (36,8% >24h), duración de VM de 6,0h (RIQ 3,7–102,0), ingreso en UCIP de 5,0 días (RIQ 2,7–18,7), media de VIS máximo de 0 (RIQ 0–14). La neopterina inicial fue de 2,3±1,2nmol/l y a las 24h de 2,3±1,4nmol/l. La biopterina basal fue 1,3±0,5nmol/l y a las 24h 1,4±0,4nmol/l. La neopterina fue significativamente mayor en estancia >6 días (p=0,02), VM>24h (p=0,023) y con complicaciones (p=0,05). La neopterina se correlaciona de forma directa con la duración de VM (rho=0,6; p=0,011), la estancia en UCIP (rho=0,75; p<0,0001) y el VIS (rho=0,73; p=0,001). Adicionalmente, la biopterina se correlaciona directamente con el PRISM (rho=0,61; p=0,008) y la cifra de leucocitos (rho=0,88; p=0,002).
DiscusiónExiste un ascenso de neopterina con mayor estancia, mayor VIS, VM más duradera y aparición de complicaciones, lo que refleja una activación del sistema inmune celular en los más graves.
During systemic inflammatory response syndrome (SIRS) situations, an increased activity in inducible NOS (iNOS) calcium independent has been demonstrated.1,2 In the last two decades, systematic studies have provided convincing evidence about several well-known markers of systemic inflammation3–6 such as C-reactive protein (CRP), amyloid A, interleukin-6, matrix metalloproteases and others, as markers of SIRS.
Activation of macrophages is a marker of chronic latent inflammation of the arterial wall, most likely as a result of the interaction between macrophages and oxidized lipoproteins. Activated macrophages are the principal source of proinflammatory cytokines such as IL-1 β and TNF-α, and they contribute to the progression and instability of atherosclerotic plaques. The activation of macrophages by interferon-γ released from activated T cells is accompanied by release of 2-amino-4-oxo-6 (d-erythro-1′,2′,3′-trihydroxypropyl)-dihydropteridine (d-erythro-neopterin).
Therefore, neopterin production appears to be closely associated with activation of the cellular immune system.7 The biological function of neopterin is not completely clear: it is associated with nitric oxide synthesis and formation of reactive metabolites of oxygen, and it may be toxic to microorganisms.8 Increased concentrations are related to endothelial damage, neoplastic and inflammatory diseases and risk for septic complications.
Neopterin monitoring has been proven helpful in the follow-up of pathological conditions associated with the activation of cell-mediated immunity,9 such as neoplasia, autoimmune diseases, organ transplantation (20–23), congenital immunodeficiencies and AIDS.10 In some publications elevated levels have been linked to the existence of viral and bacterial infections. These studies mostly comprise a small number of individuals.
Neopterin is also considered a biomarker for atherosclerotic plaque instability both in coronary and carotid arteries.11 This biological product released from activated macrophages acts as a pro-oxidant. Consequently, N is crucial in the inflammatory process and pathophysiology of the atheromatous process as well as in cellular death.12
The aim of this study is to determine the correlation between plasma neopterin and biopterin levels and severity and degree of SIRS.
Patients and methodsSingle-center prospective observational study of patients admitted to PICU between November 2014 and February 2015 who met the inclusion criteria: age 7 days to 14 years, fulfillment of the SIRS criteria and signed informed consent. Exclusion criteria were intestinal resection, abdominal radiotherapy, intestinal inflammation or necrosis (enterocolitis, mucositis, …), development of pulmonary hypertension and age less than 7 days. Plasma neopterin and biopterin levels were measured at CEDEM Laboratory (Centro de Diagnóstico de Enfermedades Moleculares, Universidad Autónoma de Madrid), using as normal levels those from non-hospitalized healthy children with no known disease and with no active infection in the previous month, according to CEDEM's data.
SIRS was considered if two or more of the following conditions13 were met:
- -
Temperature >38°C or <36°C.
- -
Tachycardia:
- •
Children <5 years: FC >180/min.
- •
Children >5 years: FC >150/min.
- •
- -
Tachypnea:
- •
Infants: respiratory rate (FR) >60/min.
- •
Children: FR >50/min.
- •
- -
PaCO2 <32mm Hg.
- -
WBC >12,000/cm3, <4000/cm3 or >10% immature forms.
We reviewed clinical data [PICU length of stay, duration of mechanical ventilation (MV), vasoactive support measured by the vasopressor inotropic score (VIS)] and laboratory values (leucocytes, uric acid, C reactive protein (CRP), procalcitonin (PCT), albumin, lactate, neopterin and biopterin). Samples for neopterin and biopterin detection were collected on dry paper S&S 903 and analyzed by high-performance liquid chromatography (HPLC), all of them at the end of the collection, performing duplicate determinations for checking test variability. The blood samples on paper were stored frozen at −20°C, keeping them dry and protected from light. Samples for other acute phase reactants were collected at admission and after 24h and 3 days.
The Pediatric Risk of Mortality (PRISM) is a prognostic scoring system that was developed to set the physiologic variables required for pediatric intensive-care unit (PICU) mortality risk assessment and to obtain an objective weighting of the variables analyzed. Results are adjusted for age (months) and expressed as percentage (risk of mortality). The severity of respiratory dysfunction was measured by PaO2/FiO2 ratios. The time-dose for dopamine, dobutamine, epinephrine, norepinephrine, milrinone and vasopressin was recorded during the first 48h. In our analysis, vasoactive inotropic score (VIS) was calculated as described by Gaies et al.14
VIS=dopamine (mcg/kg/min)+dobutamine (mcg/kg/min)+100×epinephrine (mcg/kg/min)+10×milrinone (mcg/kg/min)+10,000×vasopressin (U/kg/min)+100×norepinephrine (mcg/kg/min)
We considered as neurological dysfunction if cerebral vascular injury (imaging test) was developed, or if regional cerebral tissue oxygen saturation decreased at least 20% from baseline for 15s (cerebral desaturation). It was considered as a marker of morbidity to require at least 1 week stay in the intensive care unit. Pulmonary dysfunction was set when PaO2/FiO2 was <300 and renal dysfunction when plasma creatinine was increased more than 20% when comparing with basal. Unfavorable evolution was defined when the patient suffered from pulmonary, renal, hemodynamic (need for two or more vasoactive drugs) or neurological dysfunction (epilepsy or cerebral vascular events) or if death finally occurred. Thus, we divided patients into two subgroups (favorable evolution; unfavorable evolution).
Informed consent was obtained from all subjects’ parents. Ethical approval for this study was provided by the Ethical Committee “CEI de Centro Provincial de Málaga”. A descriptive analysis was performed, expressing continuous variables as median and interquartile range (IQ) and categorical variables as frequencies and percentages. Variables with a normal distribution and equal variances were compared between the groups with an independent sample t test. When a variable failed the normality or equal variance tests, the Mann–Whitney test was used for comparison. Comparison of means for favorable, unfavorable and control subjects was performed by means of an ANOVA test. An Spearman's ρ was used to correlate variables. Statistical significance was defined as a 2-tailed p 0.05.
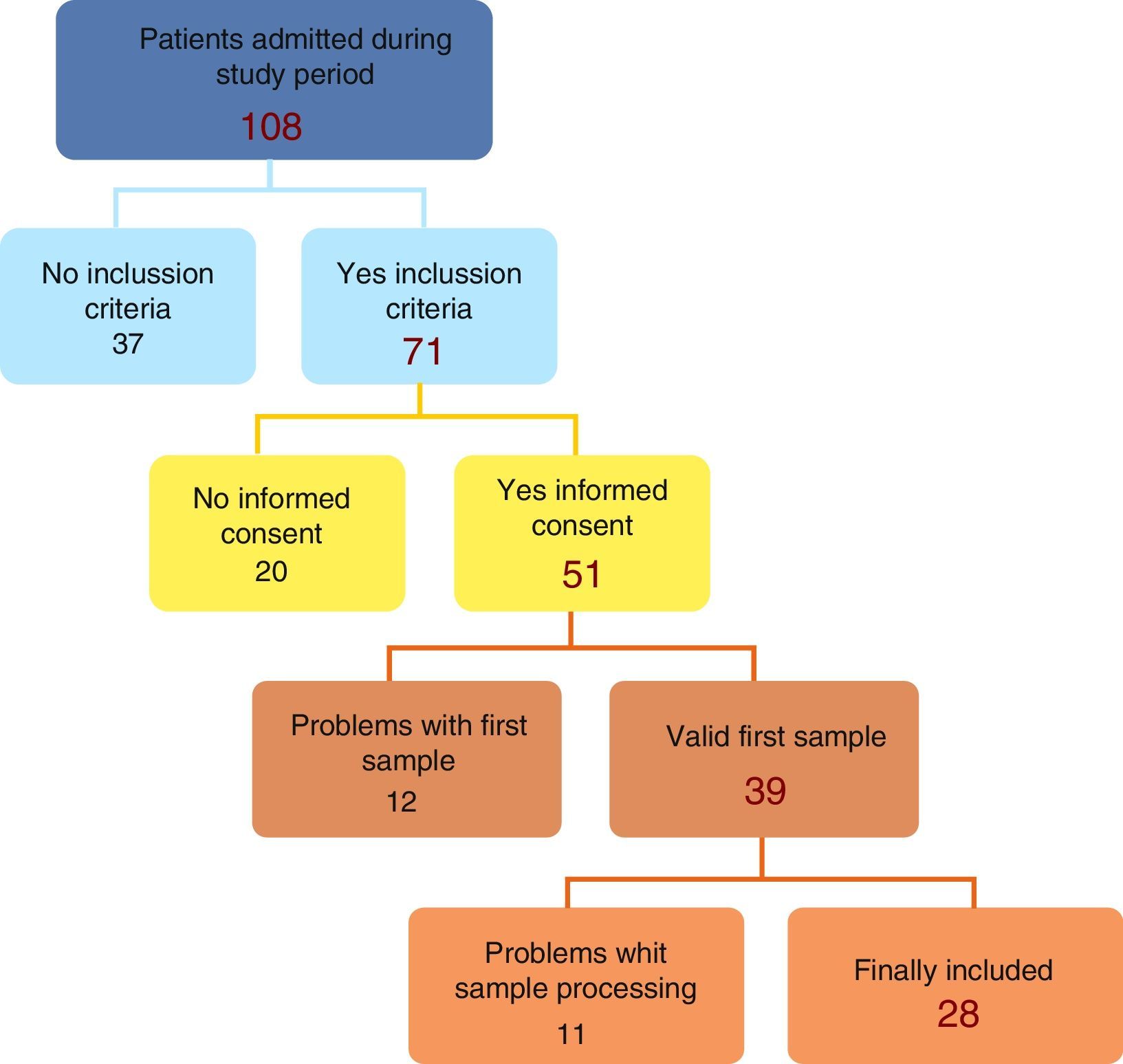
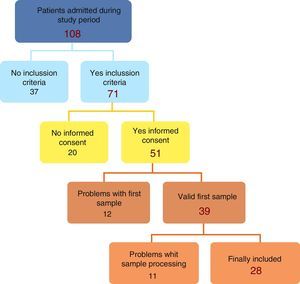
ResultsThe initial recruitment yielded 108 patients; 37 were removed due to non meeting inclusion criteria leaving a total of 71, with 28 patients finally included (Fig. 1).
A total of 28 patients were finally included: 78.9% were males, 25% less than one year-old, median age 5.04 months (IQ 1.47–10.26). The pediatric risk of mortality score (PRISM II) in the first 24h of admission was 2.0% (IQ 1.1–5.0) with an average length of stay in intensive care unit of 5.0 days (IQ 2.7–18.7) and 10 patients stayed for more than 6 days. Mechanical ventilation was used in 90.2% of patients admitted, with median duration 6.0h (IQ 3.7–102.0), only 9 patients (32%) longer than 24h; vasoactive drugs employed in 33.3%, with maximum VIS score mean 7.33±11.5 (median 0 (IQ 0–14)). Main causes of admission were cardiac surgery (39%), neurosurgery (18%), respiratory insufficiency (14%), sepsis (4%) and miscellany (25%). There were 6 patients who suffered from complications. Hemodynamic instability was the main complication (21%), followed by respiratory distress (18%), renal dysfunction (11%) and neurological impairment (3.5%). Two patients died during follow-up.
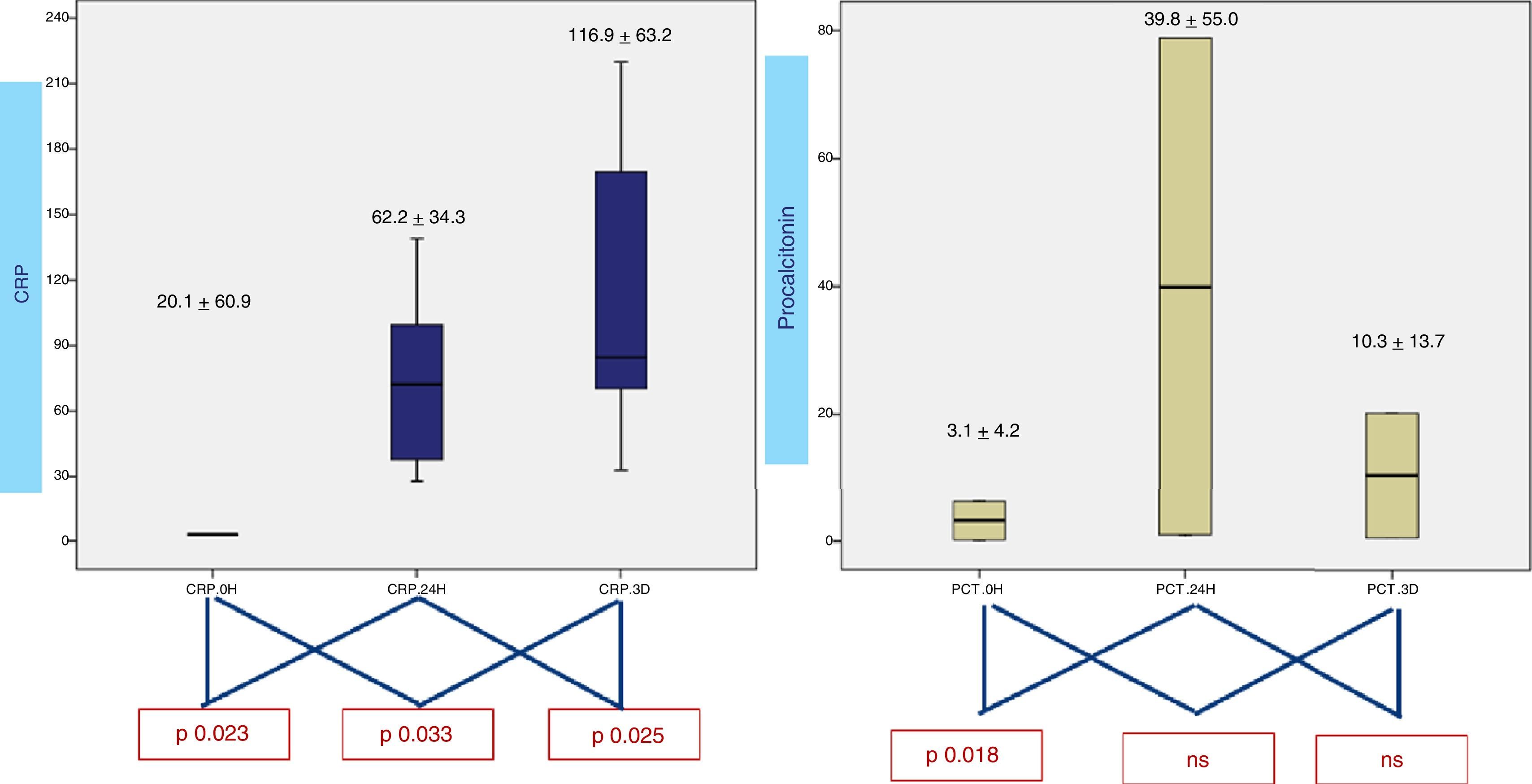
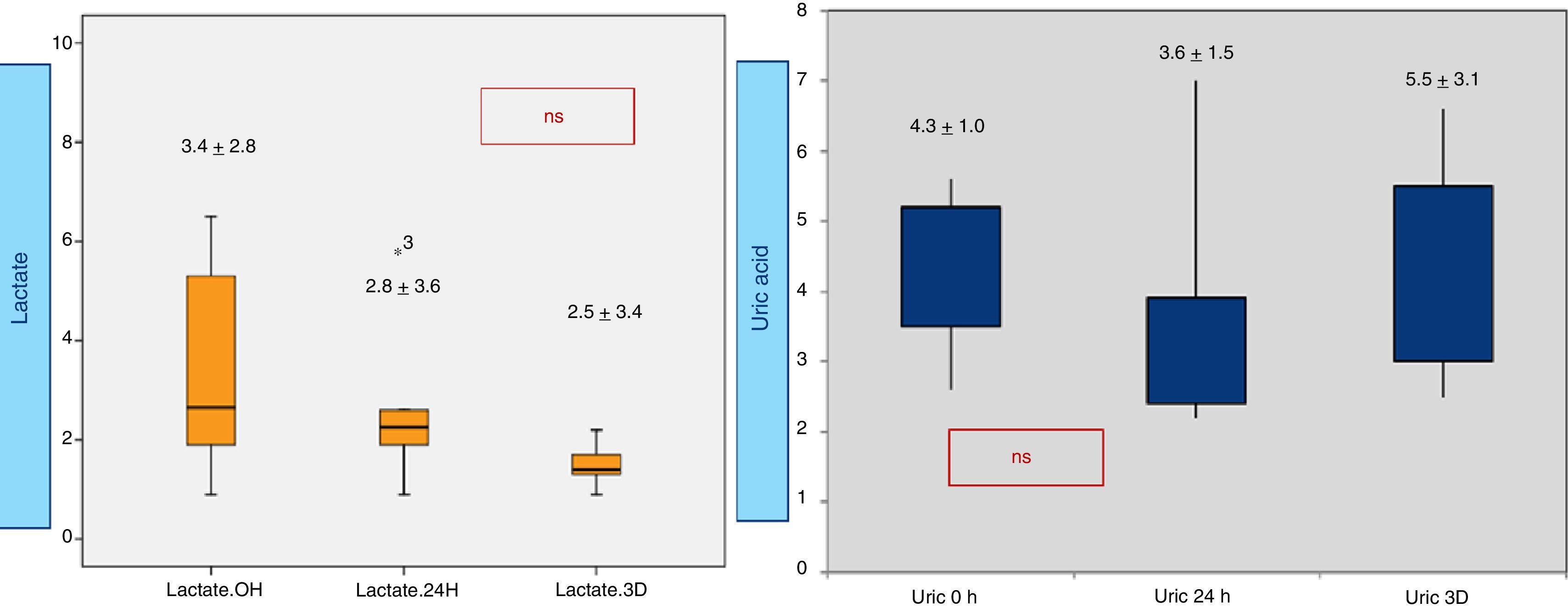
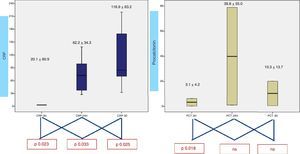
When analyzing CRP in 0h, 24h, 3rd day samples (Fig. 2), there is a statistically significative progressive increase in CRP during the first three days. Concerning procalcitonin, the increase reaches a maximum at 24h. Results from lactic acid and uric acid are shown in Fig. 3, demonstrating no significantly difference between samples.
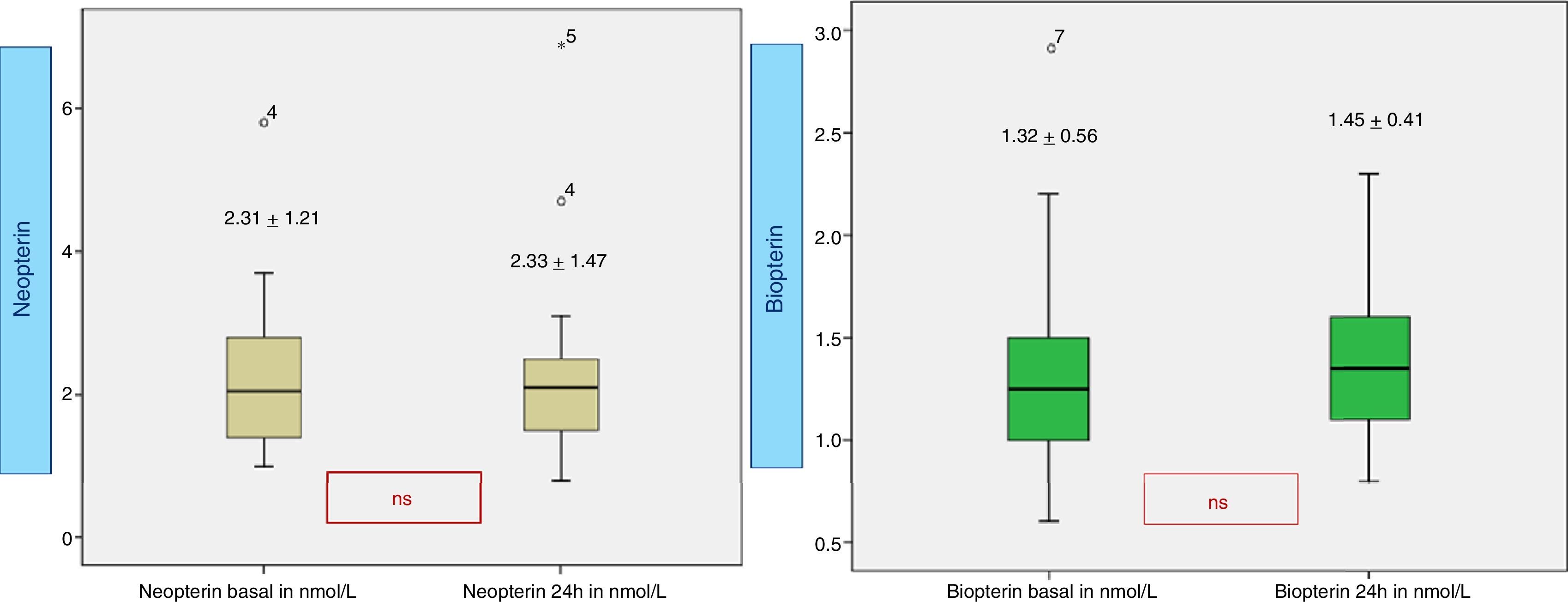
Neopterin (Fig. 4) in the early hours of admission is 2.3±1.2nmol/L and 2.3±1.4nmol/L at 24h, both of them within the normal laboratory range (0.7–3.8nmol/L). The basal biopterin is 1.3±0.5nmol/L and 1.4±0.4nmol/L at 24h (normal range 0.6–1.5nmol/L).
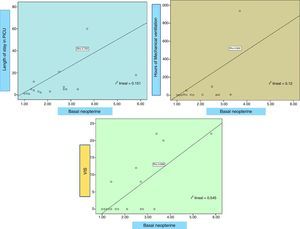
Neopterin is significantly higher in patients with length of stay >6 days (p 0.02), with mechanical ventilation (MV) for more than 24h (p 0.023) and who suffered from complications (p 0.05). Neopterin correlates directly (Fig. 5) with the duration of MV (Rho 0.6, p 0.011), length of stay in PICU (Rho 0.75, p<0.0001) and maximum VIS (Rho 0.73, p 0.001). Additionally, biopterin directly correlates with the PRISM score (Rho 0.61, p 0.008) and the leukocyte count (Rho 0.88, p 0.002). No correlation with CRP, procalcitonin, lactic or uric acid seen.
When comparing patients with favorable and unfavorable evolution, basal neopterin levels were similar (2.21±1.22 vs 2.8±1.25) but its levels at 24h were significantly different (2.0±0.98 vs 3.83±2.77, p 0.049).
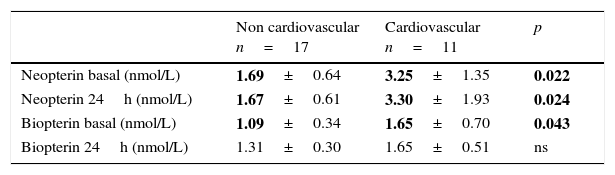
There is a clear difference between those children postoperative of cardiovascular surgery (higher neopterin and biopterin both basal and at 24h) and the rest of admissions (Table 1).
Neopterin and biopterin according to reason for admission.
| Non cardiovascular n=17 | Cardiovascular n=11 | p | |
|---|---|---|---|
| Neopterin basal (nmol/L) | 1.69±0.64 | 3.25±1.35 | 0.022 |
| Neopterin 24h (nmol/L) | 1.67±0.61 | 3.30±1.93 | 0.024 |
| Biopterin basal (nmol/L) | 1.09±0.34 | 1.65±0.70 | 0.043 |
| Biopterin 24h (nmol/L) | 1.31±0.30 | 1.65±0.51 | ns |
With a basal neopterin of 2.3nmol/L the ODDS ratio for prolonged stay is 13.3 (1.05–26.6); with a basal neopterin of 2.8nmol/L the ODDS ratio for complications is 8 (2.1–23.2) and with a neopterin at 24h of 2.35nmol/L is 13 (1.9–28.7).
DiscussionIn the present study, CRP and procalcitonin concentrations change during first three days after admission. However, concentrations started to rise in the first 6h postoperatively, reaching a peak at 24h for procalcitonin (PCT) and at 3 days for CRP, similar to that described in other studies.5,6,15
The present study was focused on the effective role played by neopterin in SIRS in pediatric patients. Unlike in other studies, ours results showed similar blood concentrations of this marker compared to the normal levels given by the laboratory11,16; however, levels were higher in patients with a longer stay in PICU, with more prolonged mechanical ventilation and a higher need for vasoactive inotropic drugs. These results must be considered useful in highlighting the activated process of macrophage cells involved in PICU pediatric patients.
We found highly significant differences between cardiovascular and non cardiovascular patients for each of the evaluated days of the observation period, similar to other data published.17 This result may be associated with increased oxidative stress caused by chronic low blood perfusion.6 On the contrary, patients with the same clinical presentation at baseline, but with low neopterin levels measured at the same time-point, did have a lower VIS. In addition to a sustained increase, patients with worse outcome showed a significantly higher level of neopterin at 24h. It has been known for several years that CPB is associated with a generalized inflammatory response. The exposure of blood cells and plasma to artificial membranes and the activation of several cell types in the setting of ischemia and reperfusion are believed to play an important role in the development of this generalized inflammatory reaction.14 Soluble adhesion molecules released into the circulation are believed to be markers of cellular activation and reflect the extent of inflammation and endothelial damage.
We were unable to establish connection with the defining parameters of SIRS: PCR, procalcitonin, uric or lactic acid. Induction of these parameters is also multifactorial; infection, severe systemic inflammation, organ dysfunction, tissue trauma, and many other aetiologies cause their induction. In certain studies, both PCT and CRP showed limited diagnostic value in critical patients in terms of identifying infectious origins.18 Several published data indicate that PCT and CRP indeed are parameters that, in particular, are more strongly induced in patients in whom the systemic inflammatory response is complicated by infection, or vice versa. CRP concentrations are high already during the less severe stages of organ dysfunction and systemic inflammation, but values are not much further increased during the more severe stages of disease.19,20
In the relatively recent point of view that ascribes to inflammation an important role in the pathophysiology of multiorgan dysfunction after cardiovascular surgery, it is intriguing to consider for neopterin an active role in the mechanisms responsible of SIRS.11,15,17
These results represent another way to demonstrate the inflammatory response in critically ill patients and the potential usefulness of neopterin as prognostic marker.
We propose that a link exists between high neopterin levels and worse SIRS profile, suggesting a potential clinical use of neopterin as a marker for disease activity in subject with cardiovascular disease. This could help in identifying patients who are at a higher risk of developing cardiovascular adverse events who might benefit from urgent preventive strategies exploitation or extensive diagnostic work-up, as well as actual therapy, depending from their co-morbidities.
Although there is much remains to be done, our work generates important findings in the field of immune mediated response to critical illness. Having acknowledged the limitations of data processing, we can nevertheless confirm that there are some limitations of this study. Although the present study has yielded some preliminary findings, its design is not without flaws. The main limitations of our study were the small sample and the high number of losses derived from blood sample collection/processing.
ConclusionsIn conclusion, neopterin can be considered as a representation of the macrophage activation process and, based on our findings, we hypothesize that plasma level of neopterin is useful to elicit the involvement of the activated macrophage, which, in turn, is able to promote oxidative stress. The plasma level of neopterin may also be considered a new and diverse target for medical and interventional procedures in PICU patients. Therefore, new and original medical protocols are required to address this issue in order to protect against the inflammatory process, with N serving as a novel marker to monitor its efficacy.
Authors’ contributionR. Gil-Gómez had primary responsibility for protocol development, patients’ enrolment, outcome assessment, preliminary data analysis and writing the manuscript.
J. Blasco-Alonso participated in the development of the protocol and analytical framework for the study, was responsible for patient's enrolment and contributed to the writing of the manuscript.
P. Sánchez and V. Rosa were responsible for an analytical framework and writing of the manuscript.
G. Milano supervised the design and execution of the study and contributed to the reviewing of the manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Gil-Gómez R, Blasco-Alonso J, Sánchez-Yáñez P, Rosa-Camacho V, Milano G. Niveles de neopterina y síndrome de respuesta inflamatoria sistémica en pacientes críticos pediátricos. An Pediatr (Barc). 2017;87:343–349.