To determine whether high levels of mid-regional pro-atrial natriuretic peptide (MR-proANP), copeptin, and procalcitonin (PCT) plasma concentrations are associated with increased mortality risk.
MethodsProspective observational study including 254 critically ill children. MR-proANP, copeptin and PCT were compared between children with high (Group A; n=33) and low (Group B; n=221) mortality risk, and between patients with failure of more than 1 organ (Group 1; n=71) and less than 2 (Group 2; n=183).
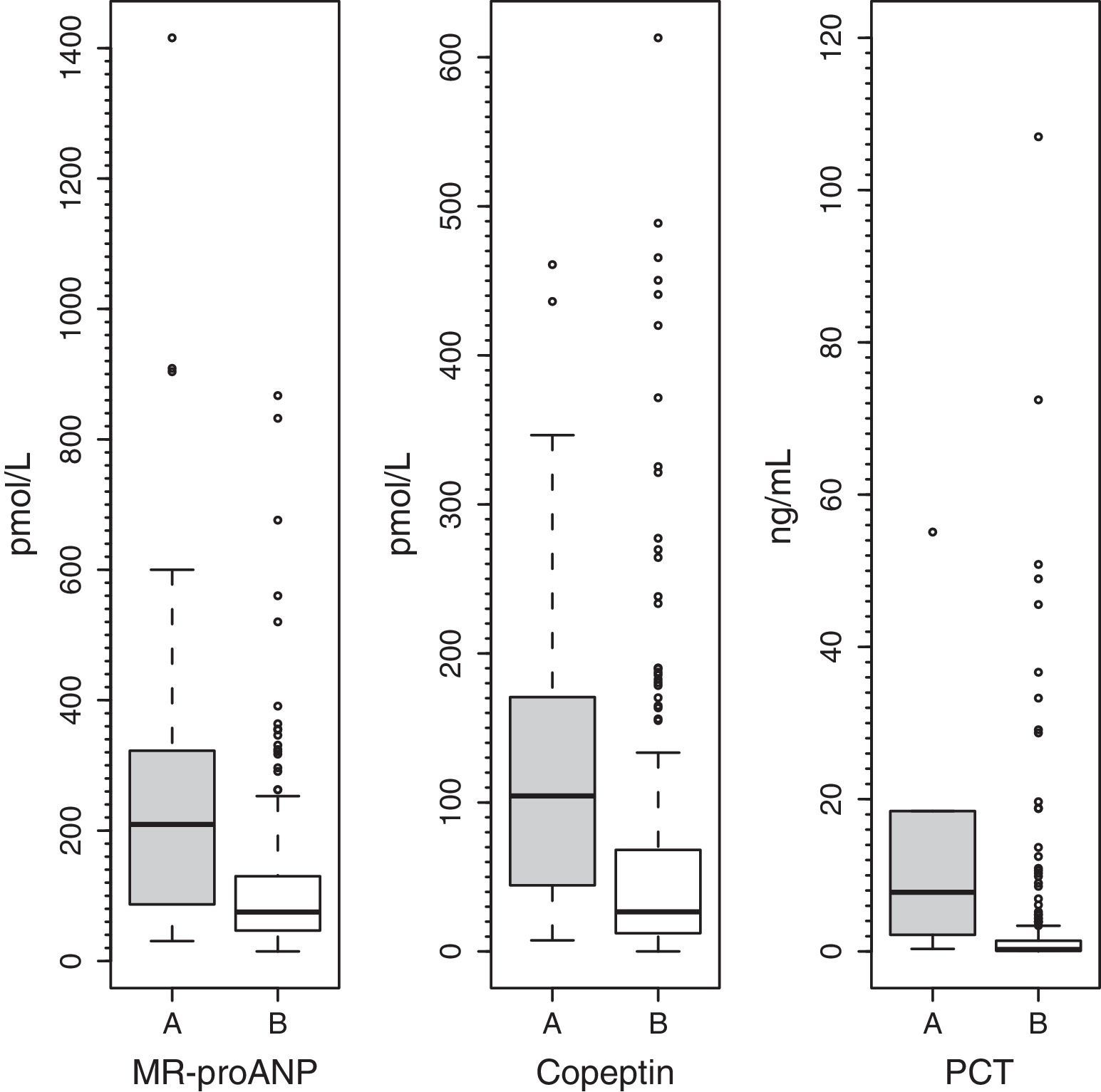
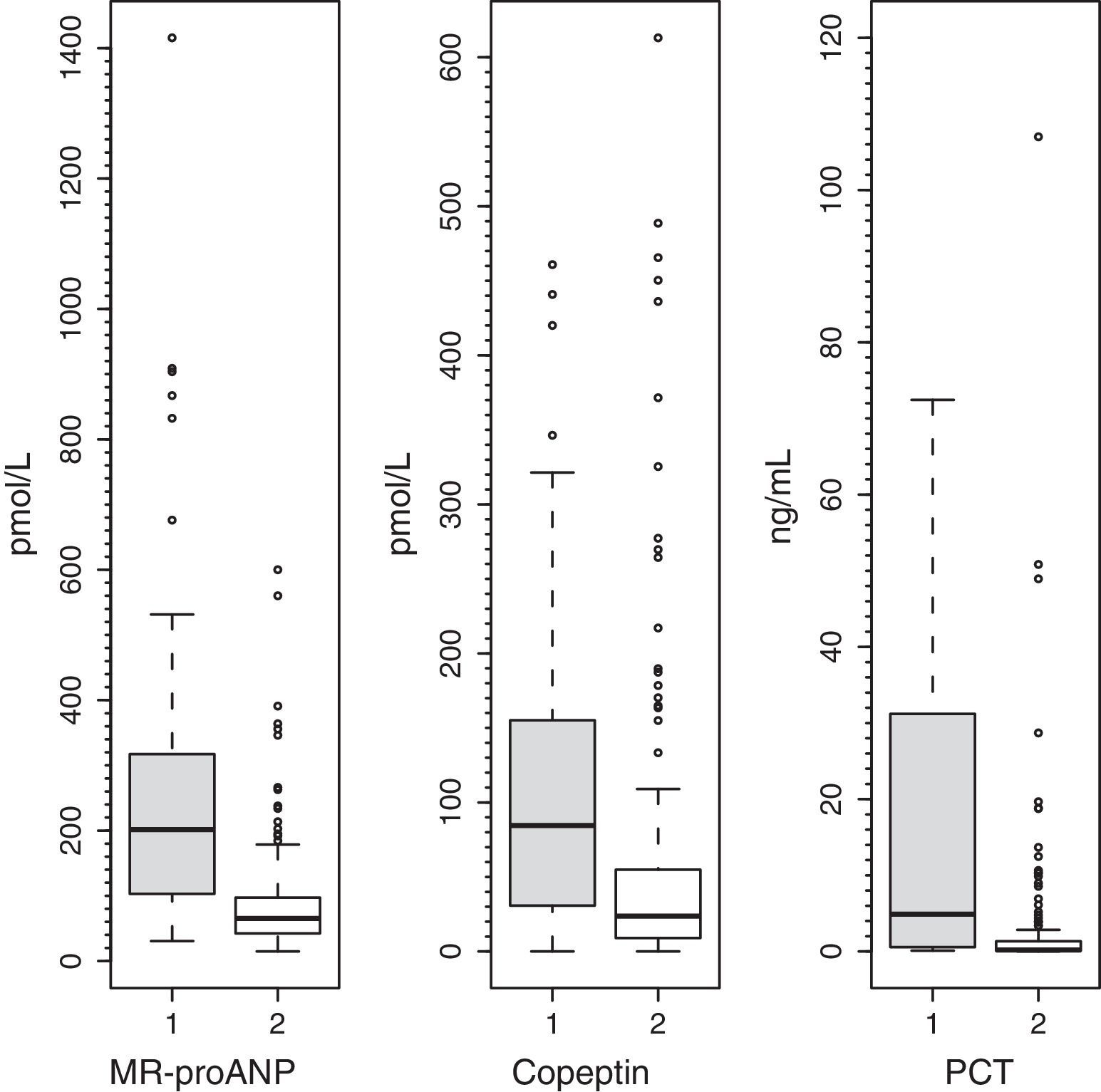
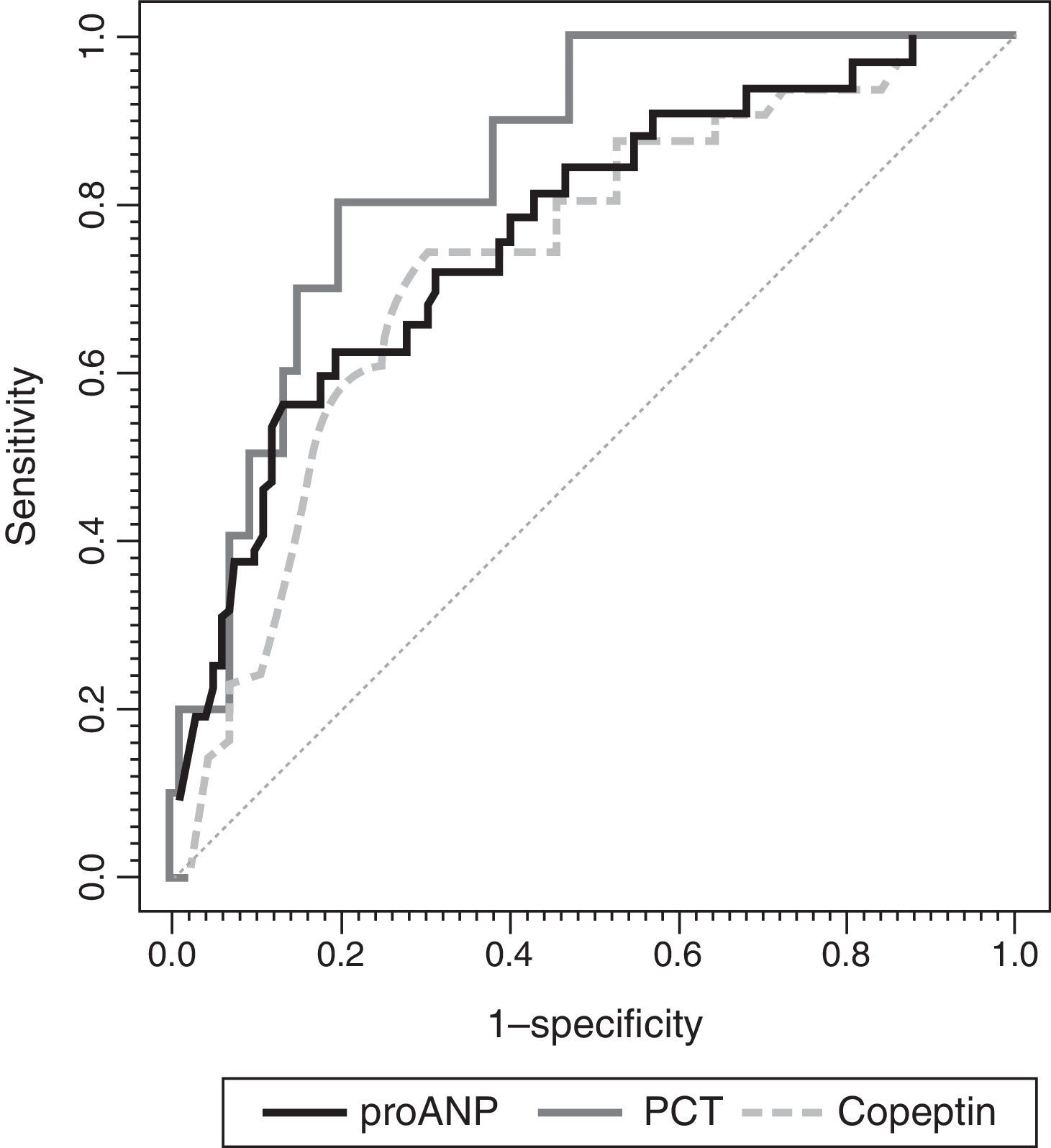
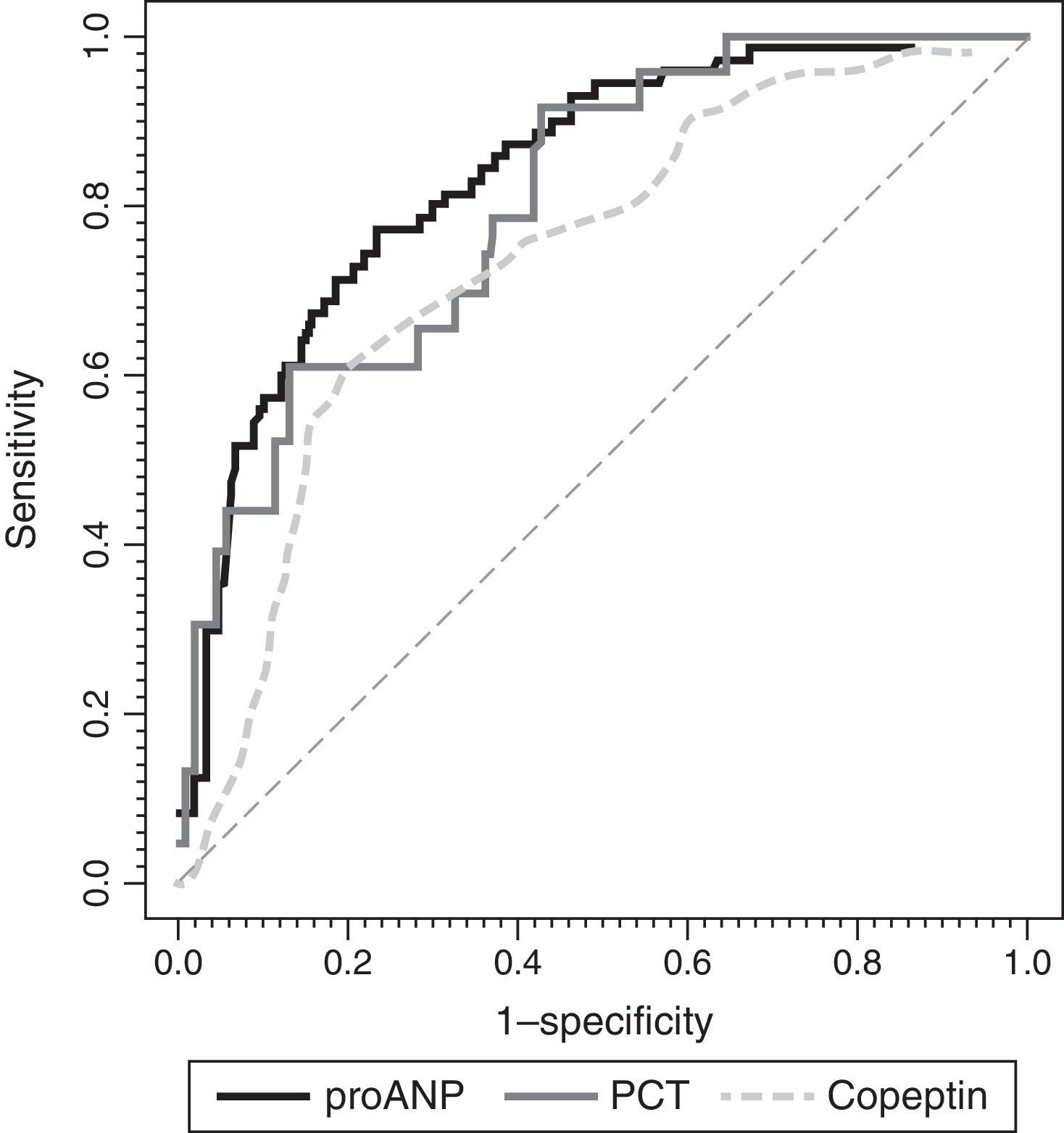
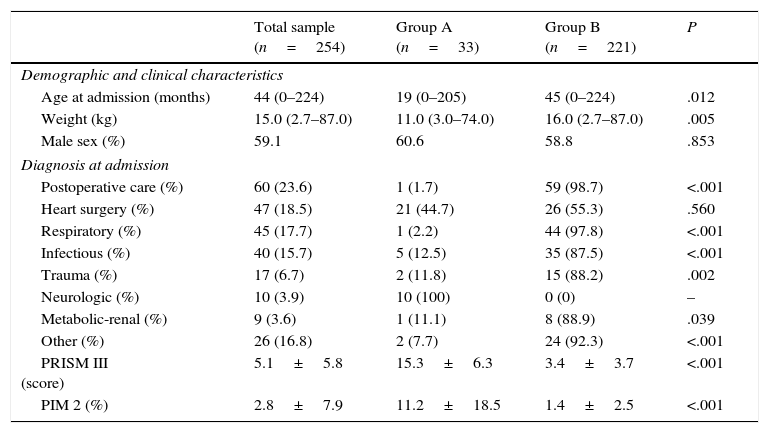
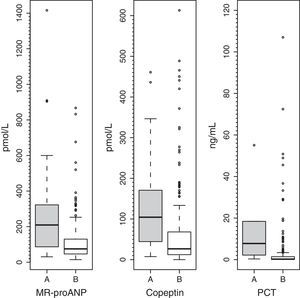
ResultsMedian (range) of MR-proANP, copeptin, and PCT levels in group A vs B were, respectively: 209.4 (30.5–1415.8) vs. 75.0 (14.6–867.2)pmol/L (P<.001); 104.4 (7.4–460.9) vs. 26.6 (0.00–613.1)pmol/L (P<.001), and 7.8 (0.3–552.0) vs. 0.3 (0.02–107.0)ng/mL (P<.001). The area under the curve (AUC) for the differentiation of group A and B was 0.764 (95% CI: 0.674–0.854) for MR-proANP; 0.735 (0.642–0.827) for copeptin, and 0.842 (0.744–0.941) for PCT, with no statistical differences. The AUCs for the differentiation of group 1 and 2 were: 0.837 (0.784–0.891) for MR-proANP, 0.735 (0.666–0.804) for copeptin, and 0.804 (0.715–0.892) for PCT, with statistical differences between MR-proANP and copeptin, P=.01.
ConclusionsHigh levels of MR-proANP, copeptin and PCT were associated with increased mortality risk scores. MR-proANP showed a higher association than copeptin with number of organs in failure.
Determinar si los niveles plasmáticos de región media del péptido natriurético proauricular (RM-proPNA), copeptina y procalcitonina (PCT) se asocian con aumento del riesgo de mortalidad.
MétodosEstudio prospectivo observacional que incluyó a 254 niños críticamente enfermos. Se compararon los niveles de RM-proPNA, copeptina y PCT entre niños con alto (grupo A; n=33) y bajo (grupo B; n=221) riesgo de mortalidad y entre pacientes con un número de órganos en fallo mayor de 1 (grupo 1; n=71) y menor de 2 (grupo 2; n=183).
ResultadosLas medianas (rangos) de RM-proPNA, copeptina y PCT en grupo A vs. grupo B fueron, respectivamente: 209,4 (30,5-1.415,8) vs. 75,0 (14,6-867,2) pmol/l (p<0,001); 104,4 (7,4-460,9) vs. 26,6 (0,00-613,1) pmol/l (p<0,001) y 7,8 (0,3-552,0) vs. 0,3 (0,02-107,0) ng/ml (p<0,001). El área bajo la curva (AUC) para diferenciar grupo A y B fue (intervalo de confianza del 95%): 0,764 (0,674-0,854) para RM-proPNA; 0,735 (0,642-0,827) para copeptina y 0,842 (0,744-0,941) para PCT, sin diferencias significativas. Las AUC para diferenciar los grupos 1 y 2 fueron: 0,837 (0,784-0,891) para RM-proPNA, 0,735 (0,666-0,804) para copeptina y 0,804 (0,715-0,892) para PCT, con diferencias significativas entre RM-proPNA y copeptina, p=0,01.
ConclusionesLos niveles elevados de RM-proPNA, copeptina y PCT se asocian con aumento de las puntuaciones de riesgo de mortalidad. RM-proPNA mostró mayor asociación que la copeptina con el número de órganos en fallo.
Determining the prognosis of a critically ill child within 12h from admission in the paediatric intensive care unit (PICU) continues to be a clinical challenge. At present, the tools used most frequently to assess risk of mortality are scales based on clinical signs and routine laboratory tests. The abnormalities found in different sections of these scales give rise to a score that correlates to mortality in patients. The scales used most frequently are the Paediatric Risk of Mortality III (PRISM III) and the Paediatric Index of Mortality 2 (PIM 2).1–5
In recent years, it has been demonstrated that there are biomarkers whose plasma levels increase in relation to disease severity. Procalcitonin (PCT) emerged as a marker of sepsis6 and later on was found to be helpful in determining disease severity and predicting patient outcomes.7 Procalcitonin could help identify children at a higher risk of mortality.8 There is also evidence that atrial natriuretic peptide (ANP) and copeptin are associated with severity in septic patients and mortality in critically ill adults.9–16 The secretion of ANP is primarily determined by increases in atrial wall tension,17 and it modulates the permeability of the endothelium, acting on the regulation of blood volume and arterial pressure.18 Arginine vasopressin (AVP) is secreted in response to osmotic or haemodynamic stimuli. Copeptin is the C-terminal portion of provasopressin.19–21 It has been regarded as an individual marker of the stress response22 that is elevated during systemic infections.23 Its levels have been associated with mortality risk in adult patients.13,24,25
Laboratory tests for measuring the levels of midregional pro-ANP (MR-proANP) and copeptin have recently become available. These peptides are synthesised from ANP and AVP, respectively, but they offer the advantage of having a longer half-life (ANP, 5–10min vs MR-proANP, 100–120min; AVP, 5–10min vs copeptin, ex vivo, several days), which makes them more suitable for everyday clinical practice.10,17,26
We thought it would be interesting to learn whether the association described in adults is also found in children, as thus far no data have been published on the association of these markers with an increased risk of mortality in paediatric patients. Thus, the aim of our study was to determine the levels of MR-proANP, copeptin and PCT in the first 12h following admission to the PICU to test the hypothesis that elevated levels of these markers could be associated with an increased risk of mortality. A secondary objective was to evaluate the hypothesis that elevated levels of these markers would correlate to a greater number of organ system failures.
Patients and methodsWe conducted an observational study in two university hospital PICUs. The study was evaluated and approved by the Research Ethics Committee of the Hospital Universitario Central de Asturias. We obtained the informed consent of all parents and participants aged more than 12 years. The sample consisted of 254 patients aged less than 19 years. Newborns were excluded. Inclusion in the study required collection of a blood sample on the basis of clinical manifestations in the first 12h from admission, and signing of the informed consent form. We collected data for the following variables: age, weight, reason for admission to the PICU, diagnosis and previous history of disease. Respiratory rate, heart rate, blood pressure, oxygen saturation, urine output and the administration of vasopressor agents were recorded every hour. Radiologic and microbiological diagnostic tests were performed as deemed necessary by the physician in charge. Blood samples for culture were collected when infection was suspected based on the clinical manifestations or the patient had a body temperature greater than 38°C. The PIM 2 score was calculated at admission and the PRISM III within 12h from admission, as is customary in clinical practice. Both scales (PIM 2 and PRISM III) had been previously validated in both PICUs.1 Samples for routine metabolic tests, including PCT levels, were collected within 12h from admission to the PICU. Samples of venous blood were collected in tubes containing ethylenediaminetetraacetic acid (EDTA). Plasma aliquots were frozen at −80°C for future measurement of MR-proANP and copeptin.
Groups by risk of mortalityPatients were divided into two groups based on their mortality risk scores. The group with a high risk of mortality (group A) included patients with PIM 2 and PRISM III scores above the 75th percentile (n=33); the group with a low risk of mortality (group B) included patients with a PIM 2 and/or PRISM III at or below the 75th percentile (n=221).
Groups by number of organ system failuresPatients were divided into two groups based on the number of organ system failures (cardiovascular, respiratory, neurologic, haematologic, renal and hepatic) following consensus criteria.27 Group 1 included patients with more than one organ system failure (n=71), while group 2 included patients with fewer than two organ system failures (n=183).
Biochemical analysis of the midregion of the proatrial natriuretic peptide and copeptinLevels of MR-proANP, copeptin and PCT were measured in plasma with EDTA using a sandwich immunoassay technique (TRACE technology; Brahms GmbH, Hennigsdorf, Germany). The detection limits were 4.3pmol/L for MR-proANP, 4.8pmol/L for copeptin and 0.02ng/mL for PCT.
Statistical analysisWe summarised the clinical characteristics of patients and biomarker values as frequencies, percentages, medians and ranges. For the comparisons of two groups of patients (A vs B and 1 vs 2), we used the Mann–Whitney U test for continuous variables and the chi-square test for categorical variables. We also calculated the diagnostic yield (receiving operating characteristics [ROC]) curves and their respective areas under the curve (AUCs) for a confidence interval (CI) of 95%. Subsequently, we compared them with the aim of determining the marker with the highest yield for the prediction of mortality risk and of organ system failure. In order to compensate for the loss of data in paired comparisons, we used a bootstrap algorithm28 to compare the AUCs. We used the Youden index to establish the cut-off point with the highest sensitivity and specificity for differentiating between the different groups (A vs B, and 1 vs 2). P values of less than 0.05 were considered statistically significant.
ResultsSample characteristicsWe included 254 patients in the study (150 male). Table 1 summarises their demographic, clinical and laboratory testing characteristics. More than half of the sample was aged less than 4 years. The most frequent reasons for admission were postoperative care, heart surgery, respiratory disease and infectious disease. A total of five patients (2%) died during their stay in the PICU. These five patients were in the group with a high predicted risk of mortality (group A). Patients in group A were younger than patients in group B.
Demographic and clinical characteristics.
| Total sample (n=254) | Group A (n=33) | Group B (n=221) | P | |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age at admission (months) | 44 (0–224) | 19 (0–205) | 45 (0–224) | .012 |
| Weight (kg) | 15.0 (2.7–87.0) | 11.0 (3.0–74.0) | 16.0 (2.7–87.0) | .005 |
| Male sex (%) | 59.1 | 60.6 | 58.8 | .853 |
| Diagnosis at admission | ||||
| Postoperative care (%) | 60 (23.6) | 1 (1.7) | 59 (98.7) | <.001 |
| Heart surgery (%) | 47 (18.5) | 21 (44.7) | 26 (55.3) | .560 |
| Respiratory (%) | 45 (17.7) | 1 (2.2) | 44 (97.8) | <.001 |
| Infectious (%) | 40 (15.7) | 5 (12.5) | 35 (87.5) | <.001 |
| Trauma (%) | 17 (6.7) | 2 (11.8) | 15 (88.2) | .002 |
| Neurologic (%) | 10 (3.9) | 10 (100) | 0 (0) | – |
| Metabolic-renal (%) | 9 (3.6) | 1 (11.1) | 8 (88.9) | .039 |
| Other (%) | 26 (16.8) | 2 (7.7) | 24 (92.3) | <.001 |
| PRISM III (score) | 5.1±5.8 | 15.3±6.3 | 3.4±3.7 | <.001 |
| PIM 2 (%) | 2.8±7.9 | 11.2±18.5 | 1.4±2.5 | <.001 |
PRISM III and PIM 2 are expressed as mean±standard deviation, diagnosis at admission as absolute frequency and percentage, and the rest of the variables as median (range).
Group A: group with highest mortality risk scores; Group B: group with lower mortality risk scores.
Plasma levels of MR-proANP, copeptin and PCT were significantly higher in the group of patients with a higher risk of mortality (group A) (Fig. 1). MR-proANP, copeptin and PCT values were significantly higher in patients with more than one organ system failure (group 1) (Fig. 2).
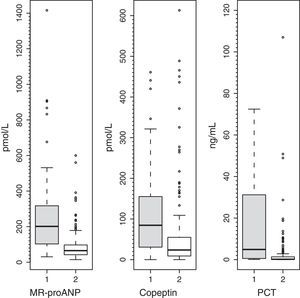
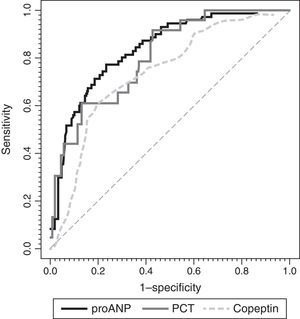
To assess the usefulness of MR-proANP, copeptin and PCT in predicting death and assessing risk of mortality in the PICU, we analysed the ROC curve for each of these biomarkers (Fig. 3). We did not find statistically significant differences between the AUCs of the three biomarkers. Levels of MR-proANP greater than 106pmol/L had a sensitivity of 72% and a specificity of 70%, while levels of copeptin greater than 64pmol/L had a sensitivity of 70% and a specificity of 74%. The optimal cut-off point for PCT to predict risk of mortality was 2ng/mL, with a sensitivity and specificity of 80%.
ROC curves for MR-proANP, copeptin and PCT for mortality risk score prediction. The areas under the ROC curve were: 0.842 (95% CI: 0.744–0.941) for PCT, 0.764 (95% CI: 0.674–0.854) for MR-proANP and 0.735 (95% CI: 0.642–0.827) for copeptin. The differences between markers were not statistically significant (MR-proANP vs PCT, P=.23; MR-proANP vs copeptin, P=.64; PCT vs copeptin, P=.14).
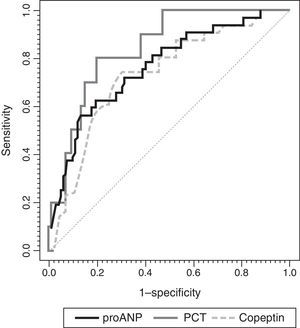
Fig. 4 shows the ROC curves for each biomarker. We found significant differences in the AUCs between MR-proANP and copeptin (P<.01). Differences in the AUCs were not significant in the comparisons between MR-proANP and PCT (P=.48) and between PCT and copeptin (P=.21). Levels of de MR-proANP greater than 101pmol/L had a sensitivity and specificity of 77%, while levels of copeptin greater than 64pmol/L had a sensitivity of 62% and a specificity of 80%. A PCT threshold of 4.1ng/mL had a sensitivity of 69% and a specificity of 88%.
ROC curves for MR-proANP, copeptin and PCT for the prediction of the number of organ system failures. The areas under the ROC curve were: 0.804 (95% CI: 0.715–0.892) for PCT, 0.837 (95% CI: 0.784–0.891) for MR-proANP and 0.735 (95% CI: 0.666–0.804) for copeptin. The difference between MR-proANP and copeptin was significant (P=.01). There were no significant differences between MR-proANP and PCT (P=.48) and between PCT and copeptin (P=.21).
Improving the assessment of the prognosis of patients admitted to the PICU is a field of research relevant to clinical practice. Some biomarkers could help stratify critically ill children based on their risk of mortality.1,8 In our study of a heterogeneous sample of critically ill children, elevated levels of MR-proANP, copeptin and PCT were associated with higher scores in mortality risk scales and a greater number of organ system failures.
The low mortality in our sample, which was similar to the rates currently reported for PICUs in developed countries, forced us to use mortality risk scores obtained using scales previously validated in our PICUs1 as the reference for the prediction of patient prognosis. This will hinder the comparison of our results with those obtained in the adult population, although it is worth noting that all patients that died belonged to the group with high mortality risk scores. The low mortality also led us to use a second severity marker, organ failure, as a reference. More than 90% of patients in the high-mortality risk group had experienced at least two organ system failures.
To date, few data are available on the potential of MR-proANP as a predictor of mortality risk, especially in the paediatric population. It has been investigated as a marker of severity in adult septic patients with promising results.9 Elevated levels of MR-proANP at admission in critically ill patients have been associated with higher mortality.9–11 In our study, we found an AUC of 0.76 for the identification of critically ill children with different predicted mortality risks. Different AUCs have been found in various studies in relation to the risk of mortality: 0.72 in the studies by Lipinska et al.10 and Nowak et al.12 0.88 in the study by Morgenthaler et al.9 and 0.89 in the study by Wang et al.29 Our cut-off point of 106pmol/L was lower than the cut-off points estimated in these adult series. This could be due to these studies having been performed with patients with different characteristics than the patients in our study, and with different methods. We studied a cohort of children and sought to differentiate the predicted mortality risk at the level of the 75th percentile, whereas the studies that we reviewed9,11,12,29,30 analysed adult populations and sought to differentiate between survivors and nonsurvivors. The study of Lipinska et al.10 only included patients with sepsis. Another study carried out in adults by Berendes et al.31 did not find an association between different levels of MR-proANP and the risk of mortality.
As for copeptin, studies on adults have found areas under ROC curves ranging from 0.70 (Seligman et al.32) to 0.87 (Du et al.14), with intermediate values of 0.75 reported by Morgenthaler et al.13 and of 0.83 by Lin et al.33 Our AUC of 0.73 is within this range. We determined a cut-off point of 64pmol/L, similar to those of 64.8 and 62.7pmol/L by Seligman et al.32 and Du et al.14 respectively. Lin et al.33 estimated a cut-off point of 52.7pmol/L, slightly below those reported in other studies. The AUC for high PCT values is also acceptable for predicting mortality risk, and it is known that PCT levels are directly correlated to severity of disease.7,34–36
As expected, the yield of MR-proANP, copeptin and PCT in identifying patients with more than one organ system failure was consistent with previous descriptions of the prediction of mortality risk in the literature. The values of the AUCs for the differentiation between groups 1 and 2 were acceptable for copeptin and good for PCT and MR-proANP (Fig. 4). For PCT, this cut-off value (4.1ng/mL) was higher than the cut-off value for the prediction of mortality risk (2.0ng/mL), while the cut-off points for MR-proANP and copeptin were similar for both predictions. Several studies have found an association between PCT elevation in the first 24h of admission37 or following surgery38 and the number of organ system failures. Lipinska et al.10 and Boeck et al.11 demonstrated the correlation between the levels of MR-proANP and the Sequential Organ Failure Assessment score. We did not find any studies that analysed the use of copeptin as a marker of multiple organ failure. In short, the information provided by these markers could improve the identification of patients with higher scores in mortality risk scales and more than one organ system failure.
The PRISM III and PIM 2 scales have been validated for the stratification of mortality risk, but are used more frequently in health administration and research than in clinical decision-making.1,3,5 Therefore, it would be helpful to have a biomarker that can be measured rapidly and provide similar information. Our group previously observed in the same cohort of patients8 that the midregion of proadrenomedullin (MR-proADM) could also be used to predict the risk of mortality (AUC, 0.866; 95% CI: 0.810–0.921) and the number of organ system failures (AUC, 0.922; 95% CI: 0.887–0.957). When we compared the AUCs, we found statistically significant differences between MR-proADM and MR-proANP (P<.05), and between MR-proADM and copeptin (P<.01). Thus, in our cohort MR-proADM had a higher predictive power than MR-proANP and copeptin. Several recent studies have also shown that MR-proADM levels could be good markers of severity in critically ill patients, both in children8,39 and adults.13 To confirm our results, future studies should assess whether RM-proADM is a better prognostic predictor than the other three markers.
There are several limitations to our study. First of all, we could not use mortality as the reference for differentiating patient prognoses because mortality was low in our sample. As an alternative, we used mortality risk scales and the number of organ system failures, while remaining aware of their limitations. Secondly, we conducted an observational study that did not allow us to draw conclusions regarding therapeutic interventions. Thirdly, our results may not be representative of other PICUs in Spain or other countries, as the sample consisted of patients from only two PICUs. The predictive power could be different for a different population. Last of all, biomarkers were analysed within 12h from admission to the PICU. Repeated measurement of biomarker levels in the early days of admission could increase their accuracy.
ConclusionElevated levels of MR-proANP, copeptin and PCT are associated with higher mortality risk scores. There are no significant differences between these three markers. We found that MR-proANP had a stronger correlation with the number of organ system failures than copeptin.
FundingThis study was partially funded by a grant from the Fundación Ernesto Sánchez Villares. The kits for the determination of MR-proANP and copeptin were provided by Brahms GmbH (Hennigsdorf, Germany).
Conflicts of interestCorsino Rey has received funds from Brahms and Thermofisher to give presentations in conferences on subjects related to biomarkers and sepsis. The rest of the authors have no conflicts of interest to declare. The Fundación Ernesto Sánchez Villares and the Brahms and Thermofisher corporations did not participate in any way in the development of the article, including the study design, data collection and analysis, writing, and decision to submit for publication.
We want to thank the medical and nursing staff of the PICUs of the Hospital Universitario Central de Asturias and the Hospital Universitario Gregorio Marañón, as well as the staff of the Department of Biochemistry of the Hospital Universitario Central de Asturias.
Please cite this article as: Rey C, García-Cendón C, Martínez-Camblor P, López-Herce J, Concha-Torre A, Medina A, et al. Asociación de valores elevados de péptido natriurético auricular y copeptina con riesgo de mortalidad. An Pediatr (Barc). 2016;85:284–290.