Eosinophilic gastroenteritis (EG) is a rare disease characterised by eosinophilic infiltration in one or more segments of the gastrointestinal tract; it has a variable presentation and its aetiology is unknown.
We have made a retrospective review of the cases of EG in patients managed at our hospital during the 2010–2015 period, analysing their clinical, endoscopic and histological characteristics, the treatment received and the long-term outcomes.
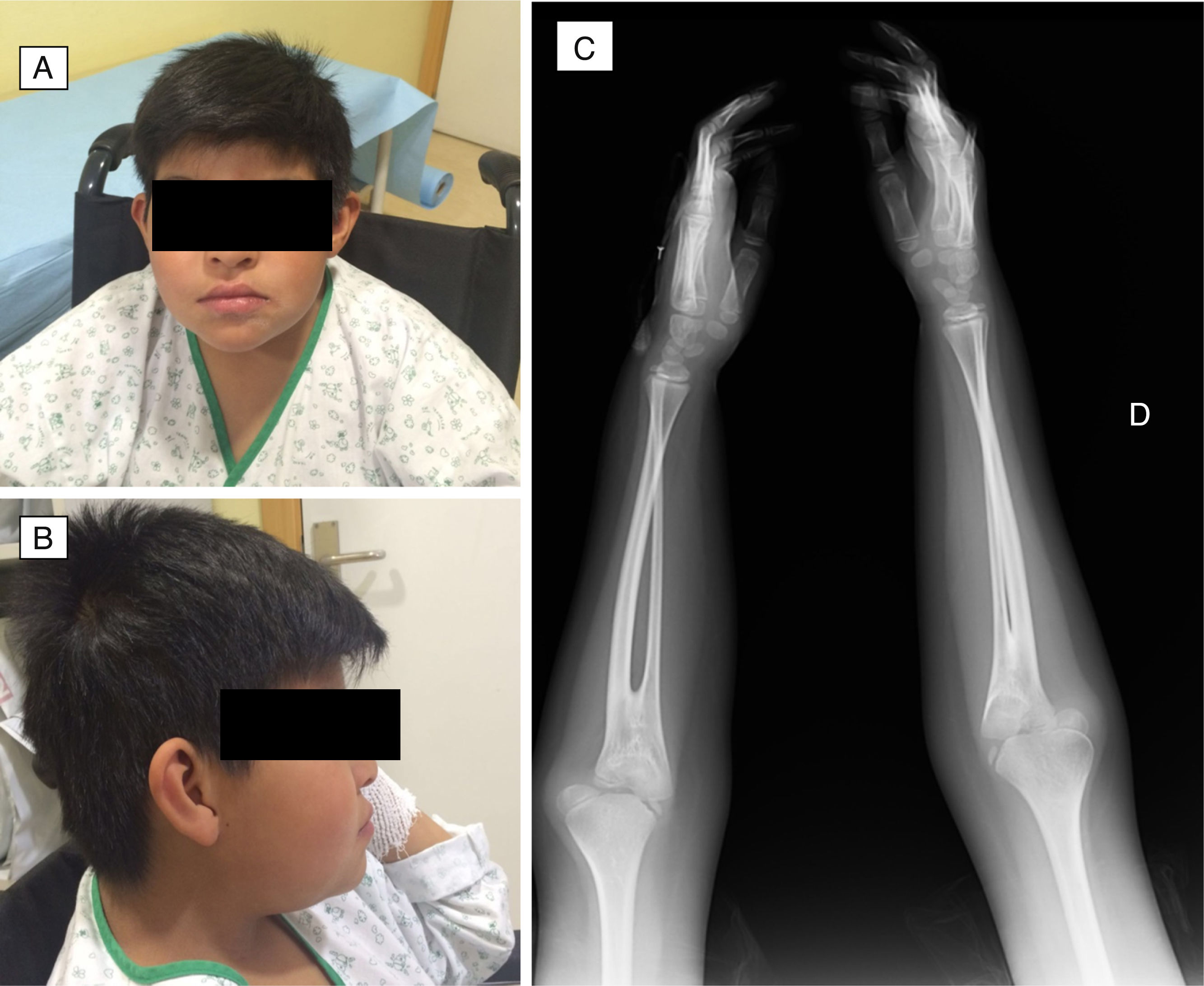
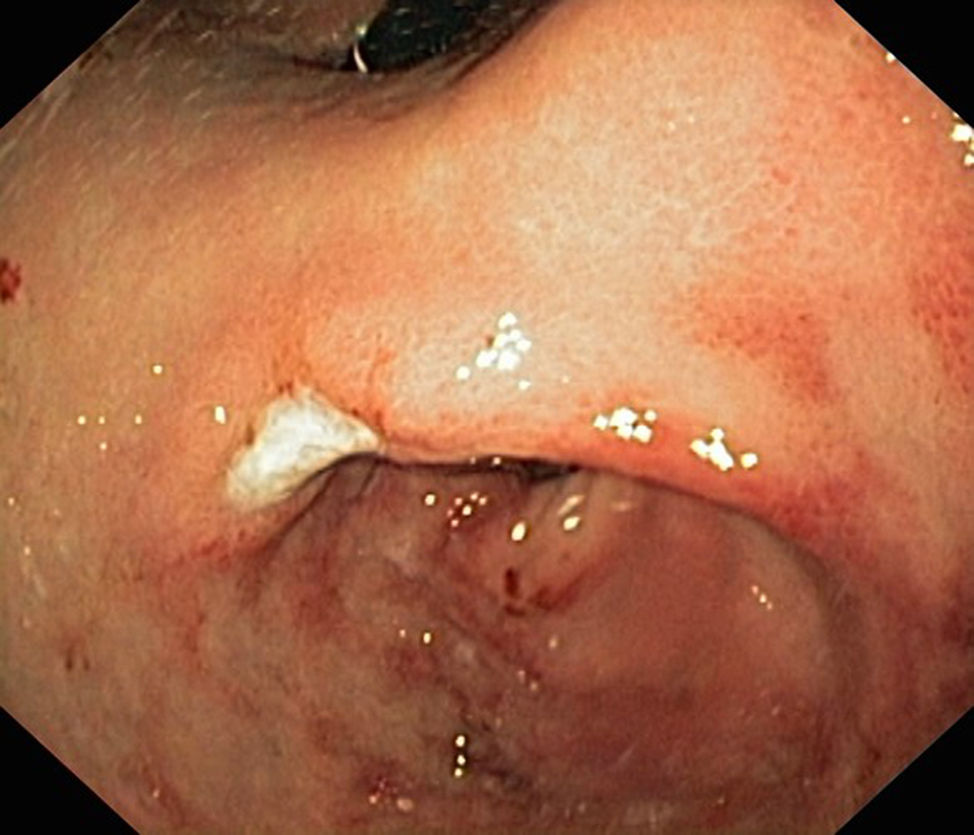
During this 5-year period, 5 patients received a diagnosis of EG. All were male, and they were aged 1–13 years. Two had underlying disease: one had undergone multivisceral transplantation, and the other had type 1 diabetes. The most frequent symptom was vomiting, found in 4 patients (80%), followed by abdominal pain in 2 (40%), faltering weight in 2 (40%), abdominal distension with ascites in 1 (20%) and diarrhoea in 1 (20%). The median duration of symptoms was 2 months (range, 1–5). All patients had serum albumin levels at the threshold of hypoalbuminaemia and 80% had peripheral blood eosinophilia. One patient had elevated IgE (111kU/L) with a positive skin test for cow's milk protein allergy. Microbiological testing was negative in all. The ultrasound examination revealed increased thickness of the gastrointestinal tract wall (at the level of the pylorus in 2, the gastric antrum and duodenum in 2, and the small intestine in 1). The endoscopic examination revealed a swollen and nodular mucosa in all patients, pyloric stenosis in 3, duodenal stenosis in 1, and duodenal ulcer in 1 (Figs. 1 and 2). Microscopic examination of biopsies revealed eosinophilic infiltration in the oesophagus in 80%, in the stomach in 100%, and in the small bowel in 60%. All patients were treated with glucocorticoids (oral methylprednisolone at 1–2mg/kg/day tapered off over 2 months) with a favourable response; the disease recurred in 3 patients, who required an additional course of glucocorticoids. As adjuvant treatment, 2 were fed with extensively hydrolysed casein formula, 2 received antisecretory drugs, and 1 underwent pyloroplasty.
Eosinophilic gastroenteritis is an uncommon disease characterised by continuous or patchy eosinophilic infiltration of the gastrointestinal tract. Its aetiology remains unknown. Several hypotheses have been proposed; the most widely accepted posits that it involves an immediate or type I hypersensitivity reaction to food allergens. We only found evidence of sensitisation to cow's milk protein in 1 of our patients. Its incidence is estimated at around 1–20 cases per 100000 births.1 Its clinical manifestations depend on the extent, location and depth of the inflammatory infiltrate. The gastric antrum and small bowel are the areas involved most frequently. It can be classified into 3 forms based on the depth of eosinophilic infiltration: mucosal disease (the most common, manifesting with abdominal pain, diarrhoea, weight loss and malabsorption), muscular layer disease (thickening of intestinal wall manifesting with symptoms of obstruction, usually at the level of the pylorus) and subserosal disease (transmural infiltration with development of eosinophilic ascites).2 Four of our patients had symptoms of obstruction, an unusual presentation,3 and one had features of ascites on ultrasound examination. Many cases also manifest with peripheral eosinophilia and protein-losing enteropathy. It is diagnosed based on the presence of gastrointestinal symptoms and histologic evidence of eosinophilic infiltration in one or more areas of the gastrointestinal tract in the absence of another cause of eosinophilia (parasitic disease, drug allergy, hypereosinophilic syndrome, coeliac disease or neoplasia). Imaging tests may reveal thickening of the mucosa and intestinal ulceration or partial obstruction. Endoscopic findings include oedema and congestion, and less frequently ulcerous lesions and narrowed areas (pylorus/duodenum).4 Microscopic examination of biopsy samples reveals a dense eosinophilic inflammatory infiltrate, with gastroenteropathy defined as presence of 25–30 or more eosinophils per high power field.5 Collection of multiple biopsy samples (at least 6) from each segment is recommended, as involvement may be patchy. Its management is based on dietary restrictions and treatment with glucocorticoids and immunosuppressive drugs.6 A high percentage of patients experience recurrence on discontinuation of steroid therapy and require an additional course of glucocorticoids or maintenance treatment with budesonide or azathioprine. Some cases of localised EG with muscle-layer involvement may require surgical correction of bowel obstruction, as was the case in one of our patients.
In conclusion, EG is an uncommon disease with nonspecific manifestations. It should be suspected in the presence of symptoms of obstruction (vomiting, abdominal distension and pain) or unexplained ascites. Peripheral eosinophilia is a frequent finding. It is a histological diagnosis, requiring biopsies from every segment of the gastrointestinal tract. Treatment with glucocorticoids is effective and safe, although symptoms often recur; furthermore, the disease may manifest with obstruction and require surgical intervention.
Please cite this article as: Tesouro Rodríguez L, Lázaro de Lucas C, Magallares García LN, Martínez-Ojinaga Nodal E, Ramos Boluda E. Gastroenteropatía eosinofílica y obstrucción digestiva. An Pediatr (Barc). 2018;88:280–281.