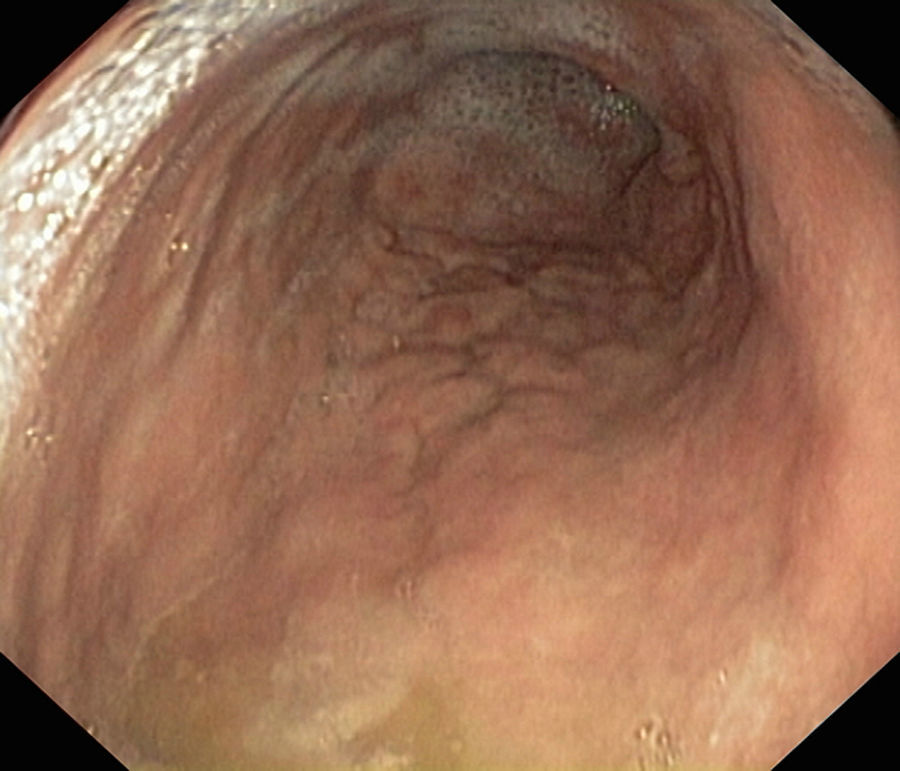
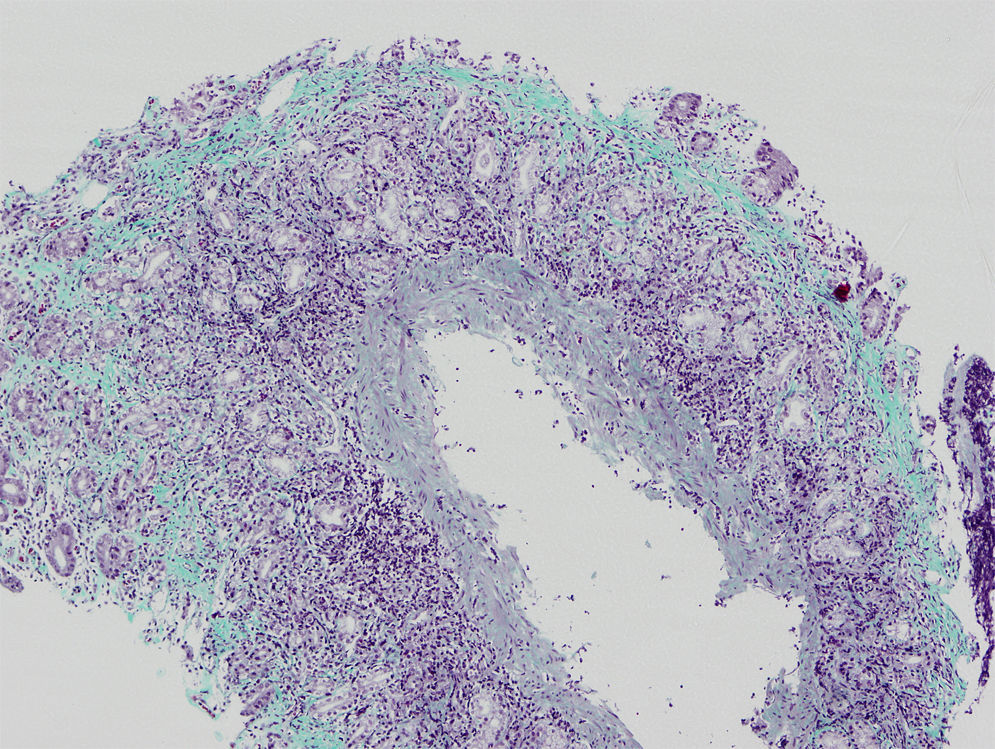
We present the case of a boy aged 11 years that sought care for postprandial feeling of fullness and nausea lasting 3 years in the absence of vomiting, heartburn or abdominal pain. The patient had normal bowel movements and development. He reported recurrent orchiepididymitis (with normal biopsy of the testes and epididymis) and a history of juvenile polyps at age 3 years. He had a family history of autoimmune disease (mother: indeterminate inflammatory bowel disease, chronic gastritis and arthritis; father: psoriatic arthritis; maternal side of family: chronic gastritis, pernicious anaemia, Sjögren syndrome and coeliac disease). The results of the diagnostic tests were: haemoglobin, 13.3g/dL; ferritin 15μg/L; vitamin B12 150pg/mL. The patient had low levels of pepsinogen I (3.5ng/mL), pepsinogen II (6.3ng/mL) and gastrin (19.6pg/mL). The results of the complete blood count, blood chemistry panel, blood clotting tests, thyroid panel and quantitative immunoglobulin tests were normal. The assays for the detection of ANA, ANCA, ASCA, antitransglutaminase IgA, anti-parietal cell, anti-LKM, anti-smooth muscle, antimitochondrial and anti-intrinsic factor antibodies were negative. The level of faecal calprotectin was normal. The upper GI endoscopy revealed thickening of the folds of the gastric mucosa with a nodular appearance (Fig. 1). Examination of the gastric biopsy revealed inflammatory cell infiltration, a thick sub-epithelial collagen band and marked oxyntic gland atrophy. There was no evidence of Helicobacter pylori infection, gastric metaplasia or epithelial dysplasia (Fig. 2). A colonoscopy ruled out large bowel involvement. The patient was asymptomatic, so he continued treatment with vitamin B12 and iron supplementation. The patient underwent follow-up endoscopic examinations at 6 and 18 months that revealed persistence of collagenous gastritis; he remained asymptomatic.
Collagenous gastritis is a rare condition. Approximately 60 cases have been described in the literature.1 The first case was described in 1989 by Colleti and Trainer in a female patient aged 15 years.2 At present, its precise aetiology and pathogenesis are not well understood. The deposition of collagen may be due to an abnormal response following exposure to a toxic or infectious agent, with chronic inflammation or increased vascular permeability that would allow extravasation and deposition of plasma proteins and collagen. It usually has onset before adolescence and it is more frequent in the female sex. It is classified into 2 possible types based on the affected region of the gastrointestinal tract: collagenous gastritis or collagenous sprue. The first type mainly affects children and young adults and involves the stomach. The second type is found in adults and involves the proximal small intestine, manifesting with watery diarrhoea and weight loss due to malabsorption. Both types may appear in association with collagenous colitis. Autoimmune comorbidities, such as thyroid disease, rheumatoid and psoriatic arthritis, systemic lupus erythematosus or Sjögren syndrome, are common. The association with autoimmune disorders is rare in paediatric patients.3,4 The study conducted by Arnason et al.5 described 3 different histological patterns: eosinophil-rich, lymphocytic and atrophic. The atrophic pattern is very rare in children. Our patient exhibited a marked atrophy of the oxyntic glands, which is characteristic of this last pattern. In patients with the atrophic presentation, the disease may have an autoimmune aetiology, although the findings in our patient did not evince the presence of autoimmune disease. The clinical presentation is nonspecific and includes abdominal pain and iron-deficiency anaemia, as well as dyspepsia and vomiting. The differential diagnosis must include gastroesophageal reflux disease, gastritis due to H. pylori and eosinophilic disorders of the gastrointestinal tract. The presence of iron-deficiency anaemia requires ruling out coeliac disease.
The diagnosis of collagenous gastritis requires performance of an upper GI endoscopy, and performance of colonoscopy is recommended to rule out colonic involvement. The findings of histological examination include a chronic sub-epithelial inflammatory infiltrate in the presence of a collagen band thicker than 10 microns. The nodular appearance of the gastric antrum and corpus is more common and characteristic in paediatric patients.5 Collagenous gastritis may also present with mucosal erythema associated with pseudopolyps, erosion, ulcers or bleeding. Its course is chronic but benign. There are no reports in the literature of progression to malignant disease, although the natural course of the disease is unknown.6
Treatment is based on proton pump inhibitors or H2 antagonists (which were contraindicated in this case, since the patient had atrophic pangastritis with hypochlorhydria) and oral iron supplementation. Anti-inflammatory drugs such as systemic corticosteroids may also be used. Hypoallergenic or gluten-free diets have been tried with little success. There is no widely accepted treatment protocol. At present, the recommended treatment consists of oral iron supplementation, and use of antisecretory or anti-inflammatory drugs for the shortest possible time is considered on a case-to-case basis.
Please cite this article as: Lázaro de Lucas C, Tesouro Rodríguez L, Magallares García LN, Martínez-Ojinaga Nodal E, Ramos Boluda E. Gastritis colágena: una forma atrófica inusual en el niño. An Pediatr (Barc). 2018;88:225–226.