Whole body plethysmography is used to measure lung volumes, capacities and resistances. It is a well standardised technique, and although it is widely used in paediatric chest diseases units, it requires specific equipment, specialist staff, and some cooperation by the patient. Plethysmography uses Boyle's law in order to measure the intrathoracic gas volume or functional residual capacity, and once this is determined, the residual volume and total lung capacity are extrapolated. The measurement of total lung capacity is necessary for the diagnosis of restrictive diseases. Airway resistance is a measurement of obstruction, with the total resistance being able to be measured, which includes chest wall, lung tissue and airway resistance, as well as the specific airway resistance, which is a more stable parameter that is determined by multiplying the measured values of airway resistance and functional residual capacity. The complexity of this technique, the reference equations, the differences in the equipment and their variability, and the conditions in which it is performed, has led to the need for its standardisation. Throughout this article, the practical aspects of plethysmography are analysed, specifying recommendations for performing it, its systematic calibration and the calculations that must be made, as well as the interpretation of the results obtained. The aim of this article is to provide a better understanding of the principles of whole body plethysmography with the aim of optimising the interpretation of the results, leading to improved management of the patient, as well as a consensus among the speciality.
La pletismografía corporal completa permite la medición de volúmenes, capacidades y resistencias pulmonares. Es una técnica bien estandarizada y ampliamente utilizada en neumología pediátrica, aunque requiere equipo específico, personal especializado y cierta colaboración por parte del paciente. La pletismografía utiliza la ley de Boyle para determinar el volumen de gas intratorácico o capacidad residual funcional, y una vez determinada esta, se extrapolan el volumen residual y la capacidad pulmonar total. La medición de la capacidad pulmonar total es necesaria para el diagnóstico de patología restrictiva. La resistencia de la vía aérea es una medida de obstrucción, pudiéndose determinar la resistencia total, que incluye la resistencia de la pared torácica, tejido pulmonar y vía aérea, y la resistencia específica, que es un parámetro más estable que corresponde al producto de la resistencia de la vía aérea por la capacidad residual funcional. La complejidad de esta técnica, las ecuaciones de referencia, las diferencias en el equipamiento, la variabilidad de la misma y las condiciones en las que se realiza han hecho necesaria su estandarización. Se analizan a lo largo del artículo los aspectos prácticos de esta técnica, especificando las recomendaciones para su realización, sistemática de calibración y los cálculos que se deben llevar a cabo, así como la interpretación de los resultados obtenidos. El objetivo de esta publicación es favorecer una mejor comprensión de los principios de la pletismografía completa con el fin de optimizar la interpretación de los resultados favoreciendo un mejor manejo del paciente y un consenso en la especialidad.
In 1956, Dubois et al. described whole-body plethysmography based on Boyle's law, according to which the volume (V) of a gas at a constant temperature varies in inverse proportion to the pressure (P) to which it is subjected, with P×V remaining constant.1,2
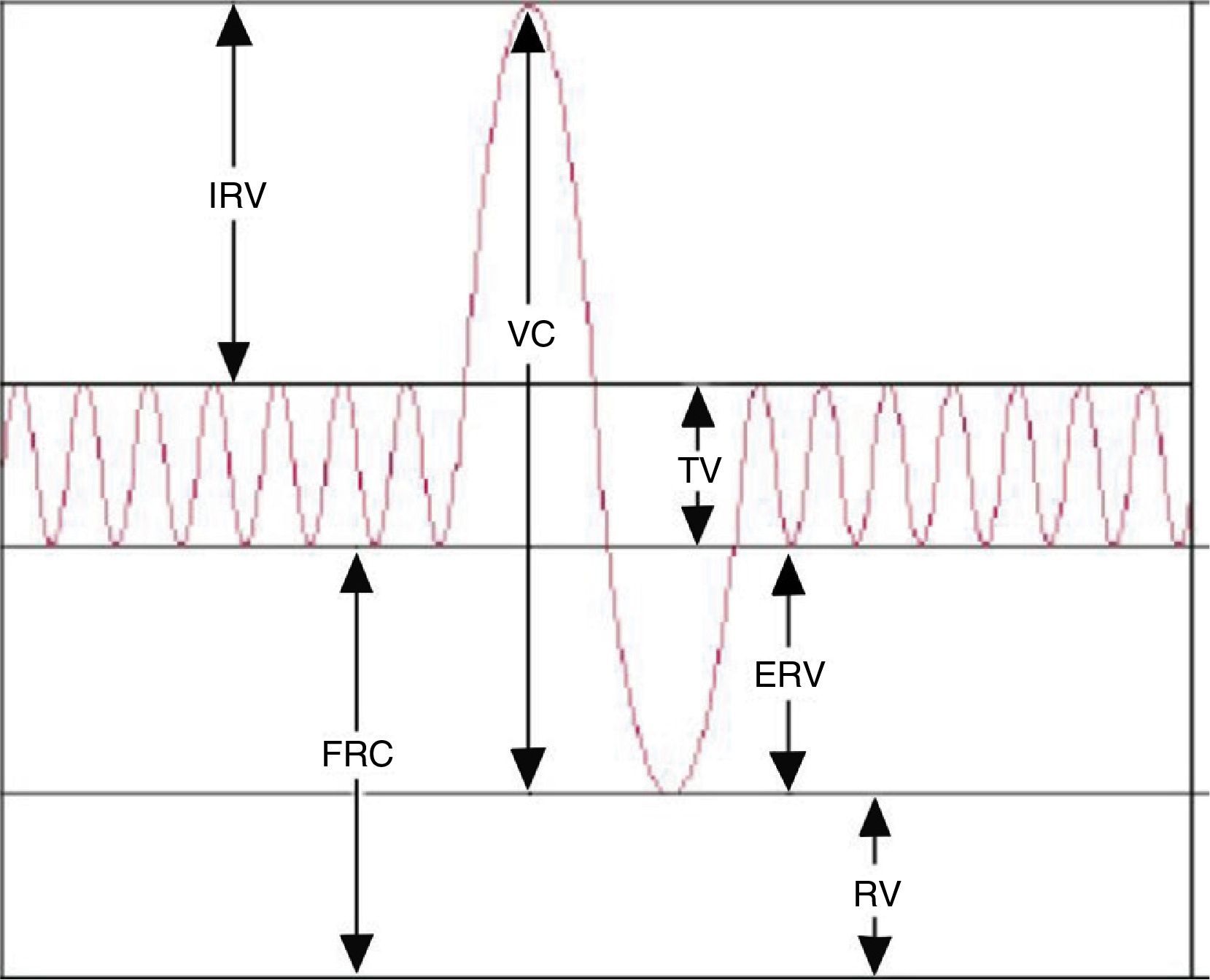
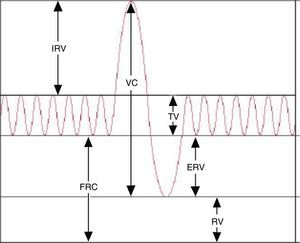
While spirometry3 is the most commonly used method to assess lung function in clinical practice, at times it is necessary to measure the volume of the air that the lungs cannot displace (static lung volumes). Thus, plethysmography remains an essential technique in the assessment of lung function. It measures several gas volumes, such as the intrathoracic gas volume (TGV) or the functional residual capacity (FRC), the residual volume (RV) and the total lung capacity (TLC).4,5 The addition of two or more lung volumes makes up a lung capacity (Table 1). This technique also measures total airway resistance (RawTOT), specific airway resistance (sRaw), airway conductance (Gaw) and specific airway conductance (sGaw).
Lung volumes and capacities.
| Capacities | ||
| Inspiratory capacity | IC | Maximum volume of air inspired after the end of expiration |
| Expiratory vital capacity | EVC | Maximum volume of air expired after a full inspiration |
| Inspiratory vital capacity | IVC | Maximum volume of air inspired after a full expiration |
| Functional residual capacity | FRC | Amount of air remaining in the lungs after expiration at tidal volume/flow |
| Intrathoracic gas volume | TGV | Plethysmography measurement equivalent to the FRC |
| Total lung capacity | TLC | Total volume of air in the lungs after a full inspiration |
| Volumes | ||
| Tidal volume/flow | VT | Volume of air inspired or expired during relaxed breathing |
| Expiratory reserve volume | ERV | Maximum volume of air that can be forcibly exhaled after a normal expiration |
| Residual volume | RV | Volume of air that remains in the lungs after a full expiration |
| Inspiratory reserve volume | IRV | Maximum volume of air that can be forcibly inhaled after a normal inspiration |
Unlike other techniques like nitrogen washout or helium dilution that underestimate the FRC because they do not measure poorly ventilated or unventilated spaces (bullae), plethysmography measures the full volume of intrathoracic gas.
There are three kinds of plethysmographs, and the one used most commonly is the constant-volume plethysmograph.4
EquipmentIt must include:
- –
Airtight chamber (2 models: older children/adults; infants).
- –
Pneumotachograph. It must meet the standards for spirometric devices (ATS/ERS 20056): capable of measuring volumes of 0.5–8.00L with an accuracy of ±3% as calibrated with a 3.00L syringe, flows between 0 and 14L/s, and recording durations of at least 30s.
- –
Shutter valve and pressure transducer to measure pressure changes at the mouth. The pressure transducer must have a sensitivity greater than 50cm H2O and a flat frequency response in excess of 8Hz. This depends on the breathing frequency during the TGV manoeuvre, which should not be greater than 1.5Hz.
- –
Pressure transducer inside the plethysmograph chamber (constant-volume variable-pressure plethysmographs). It measures the pressure within the chamber. In some systems another pneumotachograph is placed on the plethysmograph wall to measure volume changes inside the chamber (constant-pressure variable-volume plethysmographs). It must be accurate to ±0.2cm H2O.
- –
Computer, printer and weather station (depending on the equipment).
- –
Mouthpieces with disposable in-line filters 99% effective in filtering out viruses, bacteria and mycobacteria; dead space of less than 100mL and a resistance lower than 1.5cm H2O to a flow of 6L/s.
Flow metres should be calibrated following the protocol established by the manufacturer and adhering to the ATS/ERS 2005 spirometry standards.3 Plethysmographs usually have automatic calibration systems (chamber seal and transducer alignment).
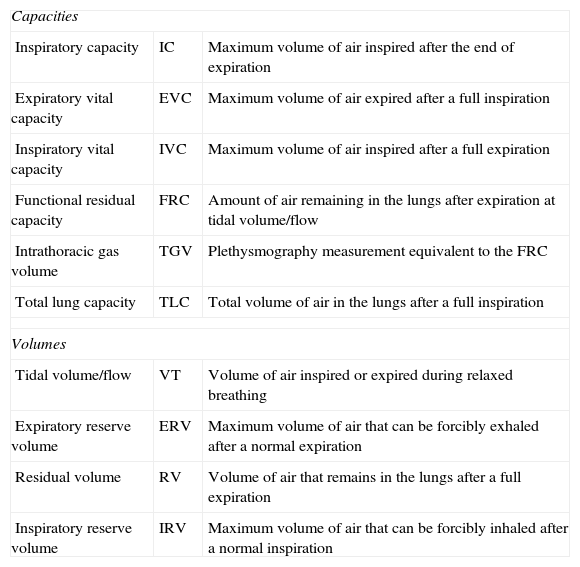
Plethysmography manoeuvre procedureIt is important to record the patient's age (years), weight (kg), ethnicity and height (cm). If the patient has difficulty standing up (chest or neuromuscular malformations) the arm span can be used instead of the height. The patient is given detailed information about the test (Tables 2 and 3). The chamber door is shut, letting 1min elapse before starting for the temperature to stabilise. The patient is instructed to breathe through the mouthpiece, supporting his or her cheeks in both hands, in small volumes and at a rate of 20–60 breaths per minute (0.5–1Hz). A set of about 10 tidal breaths should be recorded, seeking to achieve a stable FRC level (variations<100mL).
Recommendations for the technician performing the test.
| a. Always use a new mouthpiece with a disposable in-line filter for each patient |
| b. The mouthpiece must be held with the teeth and sealed with the lips, without obstructing it with the tongue |
| c. Explain how to place the hands over the cheeks to prevent leaks during the manoeuvre. Explain how to use the nose clip |
| d. Instruct the patient on how to position him or herself in the box, sitting with a straight chest and neck and with both feet resting on the floor. Check that the patient is breathing in a relaxed manner at tidal volume |
| e. Demonstrate the IVC manoeuvre, which must start with IC manoeuvres following occlusion |
Preparation of the equipment before the test.
| Assemble all components (tubes, sensors, connectors, etc.) following the directions of the manufacturer |
| Clean the flow sensors according to specification, removing potentially obstructing particles |
| Turn on equipment ahead of time to let it warm up (approximately 30min prior to the test) |
| Verify that the system has no leaks and airtight seal of door |
| Verify that the shutter responds to activation with minimal resistance |
| If the plethysmograph does not have a built-in thermometer, measure the ambient temperature before calibration and before each test |
| Set up for the average relative humidity, altitude or barometric pressure, and temperature of the location where the test is performed |
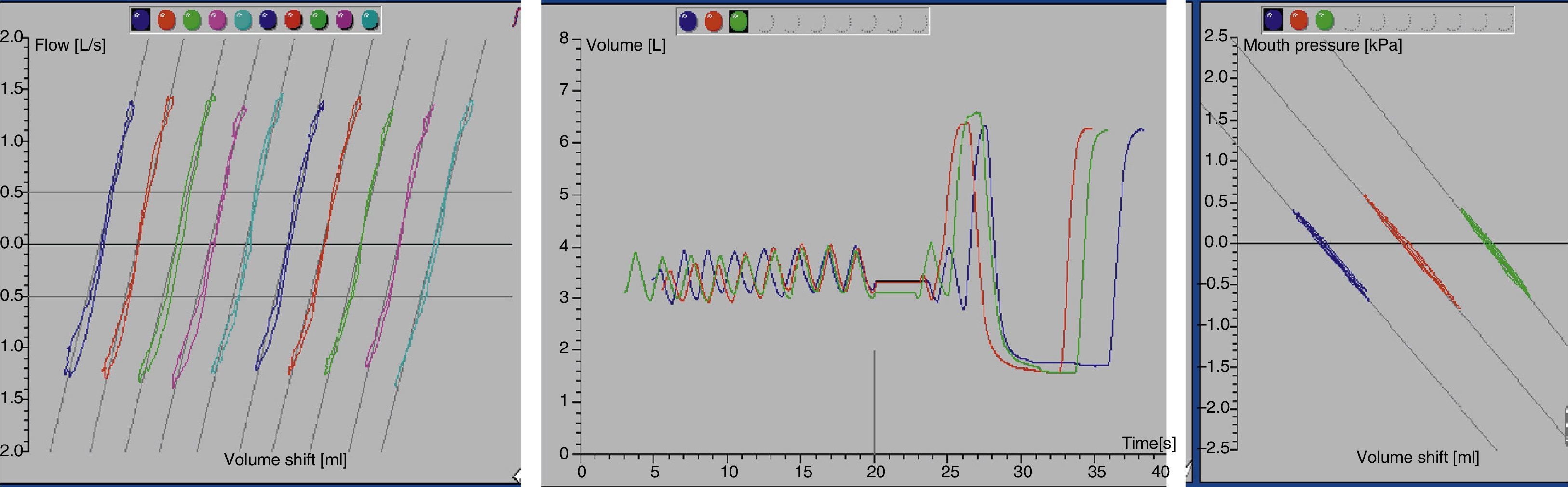
At this point, the shutter is closed at the end of an expiration (duration of occlusion, 2–3s) and the patient continues to breathe while holding his or her cheeks to avoid leaks. When the shutter is reopened, the patient has to take two or three tidal breaths followed by the slow vital capacity manoeuvre, which starts with a maximal inspiration to obtain the inspiratory capacity (IC), followed by a maximal expiration (to measure the slow vital capacity) and then a maximal inspiration (Fig. 1). If the manoeuvre fails, the technician must explain and demonstrate the test procedure to the patient one more time.
Another standardised procedure, albeit one used less frequently due to its technical difficulty, consists in having the patient exhale to residual volume after occlusion, followed by a maximal inspiration to TLC and then by a slow spirometry manoeuvre.
Plethysmography performance and quality assessmentTest quality criteriaA set of three to five technically satisfactory TGV-VC manoeuvres must be obtained. The curves must be nearly straight and superimposable on one another, and must be within the pressure calibration ranges of the transducers (±10cm H2O or 1.3kPa).
Acceptability criteriaIndividual plethysmography manoeuvres (TGV-VC) are acceptable if:
- –
Tidal breathing shows a stable FRC (at least 4 tidal breaths that agree within 100mL). This is corroborated by the graphs (Figs. 1 and 2).
- –
The difference in volume (ΔV) between the FRC level and the occlusion level is less than 200mL.
- –
The breathing frequency during shutter closure ranges between 30 and 60 breaths per minute.
- –
The plethysmograph tracing shows 3–5 TGV manoeuvres.
- –
TGV loops have consistent patterns, are free from artefacts, and show minimal hysteresis between inspiration and expiration.
- –
The two ends of the curve can be observed.
- –
The slope of the measuring line should parallel the TGV loop.
- –
The VC measurement is acceptable in relation to the highest IC or expiratory reserve volume values, must achieve a plateau of at least 1 second in duration with changes in expiratory volume of less than 25mL, and must be greater or equal than the largest FVC value obtained in the previously performed forced spirometry.
In plethysmography, these criteria should only be applied to decide when it is necessary to perform more than three acceptable manoeuvres (a minimum of three acceptable manoeuvres and a maximum of eight manoeuvres should be performed). The criteria are not to be used to exclude results from reports or subjects from a study.
The ATS/ERS 20056 requires: (a) that the three acceptable FRCpleth manoeuvres agree within 5%, and (b) that the difference between the two largest values of the repeat VC measurements be less than 150mL.
Quality controlThe chamber and volume calibrations must be performed exactly as instructed by the manufacturer. Testing of a biological control (healthy nonsmoker) should be performed at least once a month and whenever an error is suspected, measuring the TGV, RV and TLC. Values that differ by more than 10% for the FRC and TLC or more than 20% for the RV compared to previous measurements on the same subject suggest errors.
IndicationsThe main indication is the diagnosis and characterisation of restrictive ventilatory patterns (assessment of disease severity, course of disease, and response to treatment).
It can also be used to assess the severity of restriction in diseases with a mixed ventilatory pattern, and for the early detection of unventilated trapped gas compartments and airflow limitations. It allows the measurement of unventilated air compartments (subtracting the FRC measured by plethysmography from the FRC measured by helium dilution) and risk assessment for surgery (for instance, for pneumonectomy). It can be performed successfully starting at 6 years of age.
Results and reference valuesFirst, the acceptability and repeatability of the test should be assessed. The results reported once the test is deemed acceptable are the TGV (the mean of at least three TGV manoeuvres that agree within 5%), the CV (the largest value in a minimum of 3 manoeuvres with values that agree within 5%), the TLC (sum of the TGV and the highest IC value), the RV and the RV/TLC ratio.
Subsequently, resistance and TGV curves are analysed, checking that loops have a closed shape (or, if they are not, assessing for potential underlying pathologies), their angle, slope, etc. Each manoeuvre is also analysed separately to assess lung volumes; tidal volume should remain stable during the test, with a stable end expiratory level volume (EELV), proper occlusion, and correct performance of the manoeuvre consisting in an inspiration followed by a maximal expiration.
The results are reported as absolute values (l) at body temperature and barometric pressure at water vapour saturation (BTPS) conditions, rounded to two decimals; as relative values (percentage relative to the reference or theoretical value); and as z-scores (distance from the predicted value in standard deviations). Currently, the upper and lower limit of normal (LLN) (2.5th and 97.5th percentiles) are calculated, and measured values are considered clinically significant if they are outside these bounds.
There are few reference data for the paediatric age group.7 The oldest references are those provided by Zapletal8 and the most recent those by Rosenthal.9 Several studies have evinced the need to update these reference values to include children younger than 6 years and of non-Caucasian descent, as ethnicity affects lung volumes and the equations derived from both studies were based on data for healthy white children. Africans have smaller lung volumes, probably because their limbs are long and their trunks short. It has also been noted that previous equations have been derived from panting manoeuvres, so they tend to overestimate FRC values, something that has a lesser impact on the determination of RV and TLC.
Interpretation of resultsThe recommendations of the ATS/ERS for the interpretation of lung function tests10 define restrictive abnormalities as reductions of VC and TLC below the LLN, applying the reference values published in the literature.7 When relative values are used, TLC, FRC and RV are considered normal when they range between 80% and 120% of the predicted value, and considered pathological when the TLC is below 80%, with the restrictive pattern being categorised depending on this percentage into mild (70–80%), moderate (60–69%) or severe (<59%). Furthermore, the pattern is diagnosed as a mixed abnormality (combination of obstructive and restrictive) when the FEV1/VC ratio and the TLC are below the LLN. The possible causes of a restrictive pattern are neuromuscular disease, kyphoscoliosis, interstitial lung disease and pneumonectomy. The ATS/ERS recommendations10 also classify patterns with a reduced VC, a normal FEV1/VC ratio and a normal TLC as obstruction, although this algorithm has been disputed, and patterns where the FRC, RV and TLC are above 120% and the RV/TLC ratio is above 20–35% as hyperinflation (when the TLC is normal the pattern suggests air trapping). In the paediatric age group, variable parameters must be interpreted with caution, such as the RV/TLC ratio (percentage of the TLC occupied by gas that cannot be exhaled, the RV). This variability results from the changes in respiratory tract characteristics that occur during growth, such as the shape and size of the thoracic cage and respiratory muscle function. Furthermore, the rapid increase in height that occurs in adolescence is not proportional to increases in thorax dimensions or changes in respiratory mechanics.
Measurement of specific airway resistancesIntroductionAirway resistance is defined as the relationship between airflow in the respiratory tract and the pressure required to generate this flow. The RawTOT value comprises the resistance produced by the chest wall, the lung tissue and the airway. The specific airway resistance (sRaw) is the product of the airway resistance and the FRC.6
Methodology and quality criteriaExpressing the results as the specific resistance (sRaw=Raw×TGV) or its reciprocal (sGaw=1/sRaw) can be advantageous if there is poor transmission of alveolar pressure, as the TGV is overestimated in the same proportion as the Raw is underestimated.11
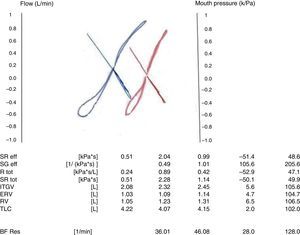
The relationship between pressure changes in the chamber (proportional to changes in alveolar pressure and airflow) can be measured when the shutter is open. This relationship (ΔPbox/V) can be represented graphically as an S shape. Once the shutter closes, the relationship of the changes in chamber pressure and mouth pressure is calculated. When the test is performed, the technician watches the display in real time. Since measurement of Raw involves inspiratory and expiratory flows, the display allows the calculation of inspiratory and expiratory resistances, which are the same in healthy individuals, but may differ in patients with obstruction.12
The chamber and pneumotachograph in the plethysmograph must be calibrated daily. The parameters obtained must be adjusted under BTPS conditions. The manoeuvre can be performed at tidal volume using a heated rebreathing bag, which is considered the gold standard, or automatically by electronic compensation.13
The flow-pressure curves are displayed in real time in the computer screen, allowing the technician to eliminate curves that have artefacts. The curves must have a similar size and shape, be parallel, and be close to zero flow. The tangent selected automatically by the computer system must be used.
To guarantee the reproducibility of the technique, at least 3 FRC measurements must be acquired that agree within 5%, and the median of three technically acceptable sets of 10 breaths shall be reported. The curves of as many breaths as possible must be obtained for the sRaw, ideally between three and five sets of five to ten breaths, depending on the software used.14,15
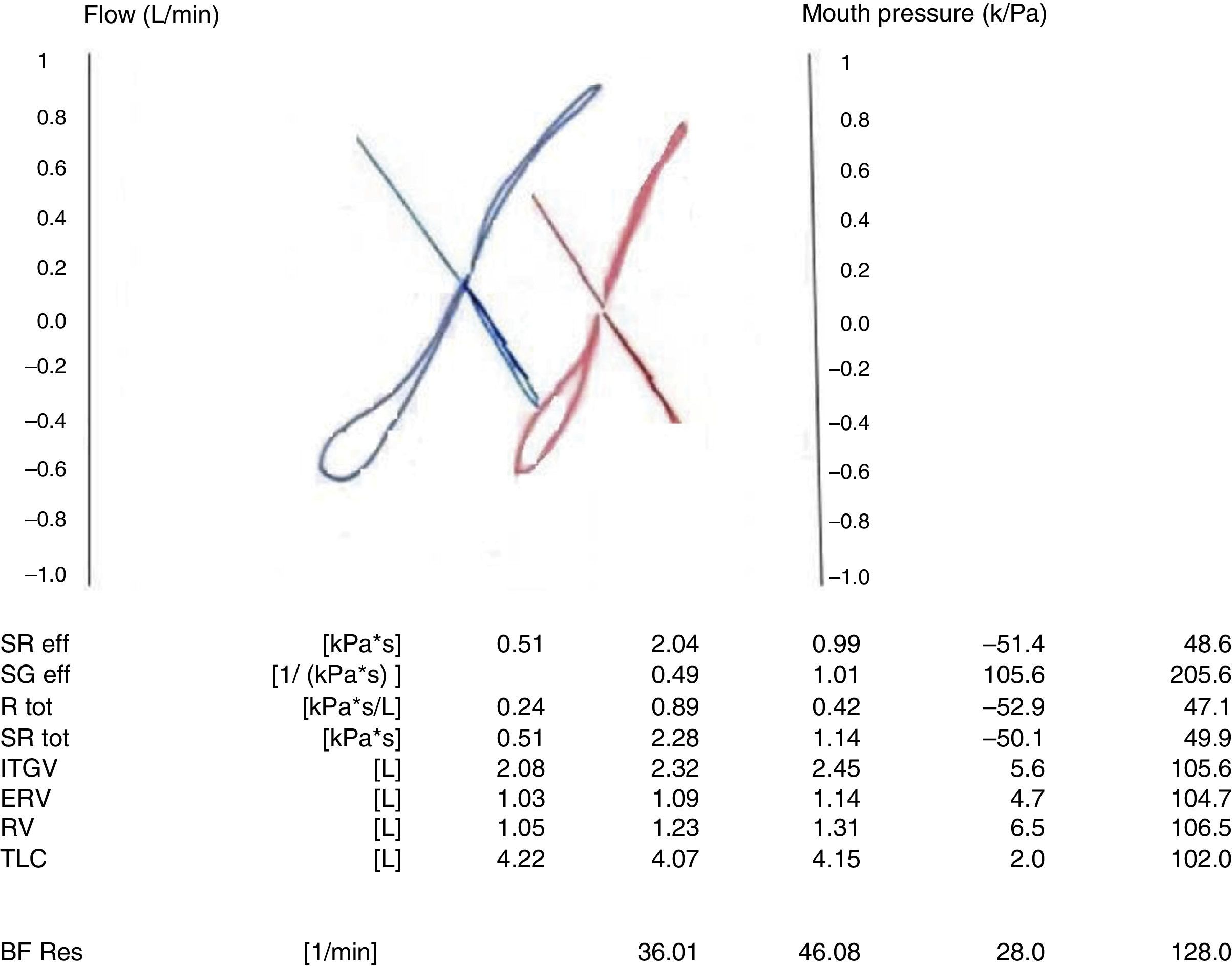
Interpretation of resultsThe sRaw is a parameter of airway obstruction. The shape of the curve provides information on the location of obstruction.15 If the patient has an expiratory obstruction, the curve takes the shape of a golf club (Figs. 3 and 4). A “cursive S” shape signals a mild diffuse obstruction; an increased inspiratory resistance is indicative of extrathoracic obstruction; an increased expiratory resistance denotes chronic obstructive pulmonary disease; and the increase of both resistances is indicative of tracheal obstruction. In generalised obstructive lung disease, there is an increase in sRaw, FRC and RV values accompanied by a reduced tidal flow.6 TLC changes may be very mild in mixed abnormalities, so measurement of carbon monoxide diffusion capacity is useful in these patients.12
Recently, it has been reported that a 42% reduction from baseline in the sRaw is statistically significant to assess the response to bronchodilators, with a 55% sensitivity and a 77% specificity.16 Furthermore, the sGaw is very sensitive to changes in airway calibre, and a 40–56% increase has been established as the cut-off point for a positive response,6,17,18 although sGaw has a lower specificity than the FEV1. Furthermore, an increase twice the baseline in the sRaw is considered a positive response to the bronchial challenge test, as is a 35–40% decrease in the sGaw.8
Indications and clinical applicationThe sRaw is the product of the airway resistance by the FRC.19 As children grow, resistances decrease and volumes increase, but specific resistance remains stable irrespective of age, sex and height. It is a sensitive and reproducible parameter for discriminating between normality and disease, and also facilitates the longitudinal interpretation of different measurements in a single individual.20–23 There is evidence of its usefulness in the clinical monitoring of cystic fibrosis and asthma,24 and also in diagnosing asthma.25 In children with cystic fibrosis, the sRaw is more sensitive than resistances measured with the interrupter technique or impulse oscillometry.19
Some authors have noted its usefulness for monitoring the response to treatment of asthmatic children.26,27 It has also proven useful in the assessment of bronchodilator reversibility and bronchial hyperresponsiveness.16
The sGaw is more sensitive than the sRaw for detecting central obstruction, and even more sensitive than the FEV1 obtained by means of forced spirometry. However, it is less reproducible than the sRaw, so a larger number of measurements need to be acquired.13 It may be more sensitive than FEV1 in the detection of airflow limitation in bronchiolitis obliterans, in which obstruction of the peripheral airways predominates,11,28 and also in asthmatic patients with moderate obstruction. It is also more sensitive in the assessment of the upper respiratory tract in vocal cord paralysis or dysfunction.29
Reference valuesSeveral published studies have provided sRaw reference values for children, including preschoolers.10,14,30 However, most researchers recommend assessing normal values in each lung function laboratory to allow inter-centre comparison studies.31
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: de Mir Messa I, Sardón Prado O, Larramona H, Salcedo Posadas A, Villa Asensi JR, Representing the Grupo de Técnicas de la Sociedad Española de Neumología Pediátrica. Pletismografía corporal (i): estandarización y criterios de calidad. An Pediatr (Barc). 2015;83:136.e1–136.e7.